

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM506772 (US11046651, Compound 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM506778 (US11046651, Compound 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM506781 (US11046651, Compound 17) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM506782 (US11046651, Compound 6) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM506783 (US11046651, Compound 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM506790 (US11046651, Compound 10) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM506794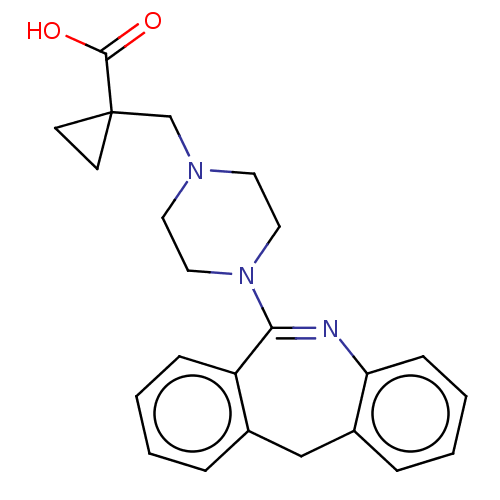 (US11046651, Compound 12) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506772 (US11046651, Compound 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506773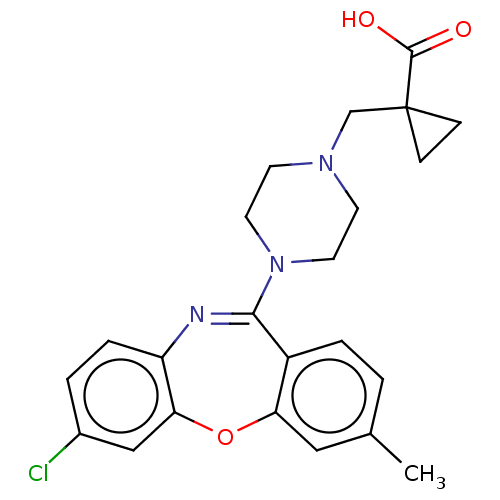 (US11046651, Compound 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506774 (US11046651, Compound 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506775 (US11046651, Compound 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506776 (US11046651, Compound 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506777 (US11046651, Compound 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506778 (US11046651, Compound 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506780 (US11046651, Compound 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506781 (US11046651, Compound 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506782 (US11046651, Compound 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506784 (US11046651, Compound 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506785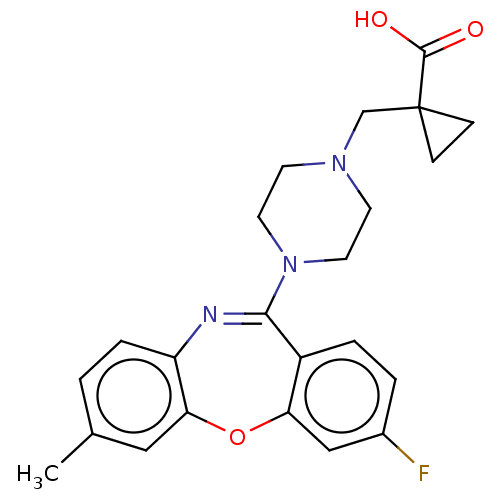 (US11046651, Compound 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506788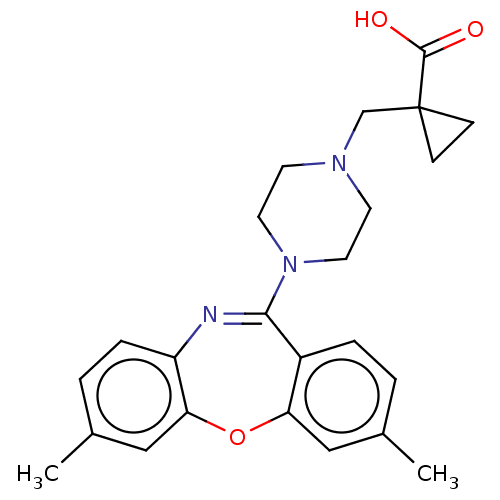 (US11046651, Compound 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506790 (US11046651, Compound 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506792 (US11046651, Compound 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM506798 (US11046651, Compound 29) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μL of a compound of the present disclosure the reference compound (Table J) was transferred to an assay plate. 1 μL of 0.2 mM SB-206533 w... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM506799 (US11046651, Compound 31) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μL of a compound of the present disclosure the reference compound (Table J) was transferred to an assay plate. 1 μL of 0.2 mM SB-206533 w... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM506800 (US11046651, Compound 33) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μL of a compound of the present disclosure the reference compound (Table J) was transferred to an assay plate. 1 μL of 0.2 mM SB-206533 w... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM506801 (US11046651, Compound 34) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μL of a compound of the present disclosure the reference compound (Table J) was transferred to an assay plate. 1 μL of 0.2 mM SB-206533 w... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM506793 (US11046651, Compound 38) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μL of a compound of the present disclosure the reference compound (Table J) was transferred to an assay plate. 1 μL of 0.2 mM SB-206533 w... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM506795 (US11046651, Compound 39) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μL of a compound of the present disclosure the reference compound (Table J) was transferred to an assay plate. 1 μL of 0.2 mM SB-206533 w... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM506797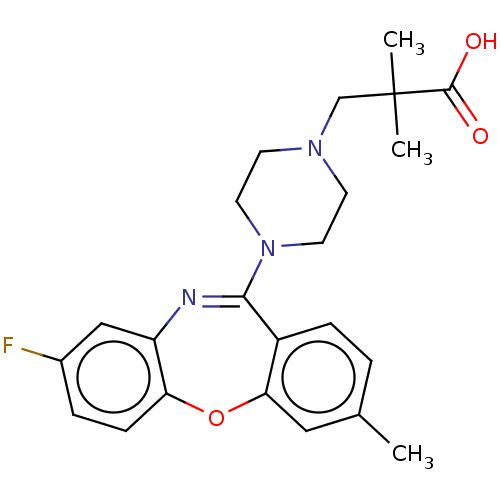 (US11046651, Compound 28) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 37.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μL of a compound of the present disclosure the reference compound (Table J) was transferred to an assay plate. 1 μL of 0.2 mM SB-206533 w... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM506776 (US11046651, Compound 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 37.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM506786 (US11046651, Compound 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 37.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM506785 (US11046651, Compound 19) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 37.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM506802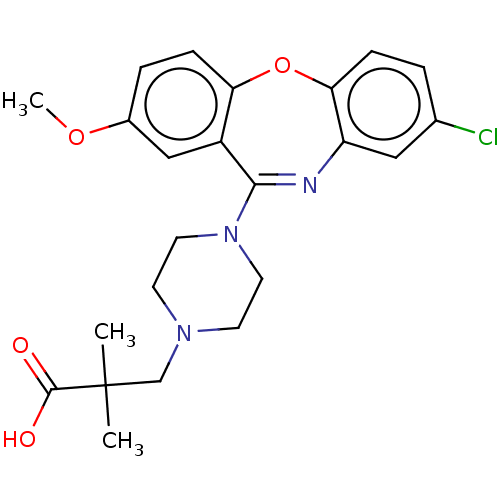 (US11046651, Compound 35) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 37.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μL of a compound of the present disclosure the reference compound (Table J) was transferred to an assay plate. 1 μL of 0.2 mM SB-206533 w... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM506774 (US11046651, Compound 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 37.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506789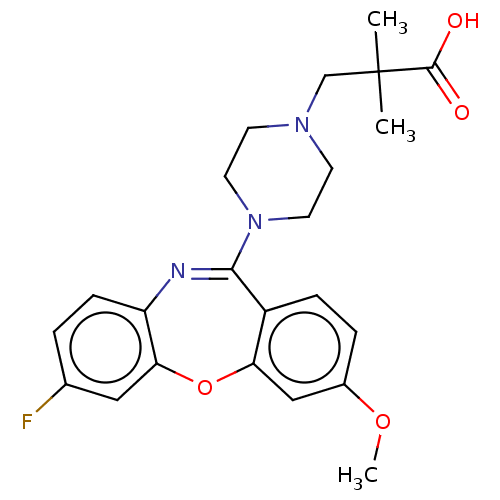 (US11046651, Compound 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 37.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506787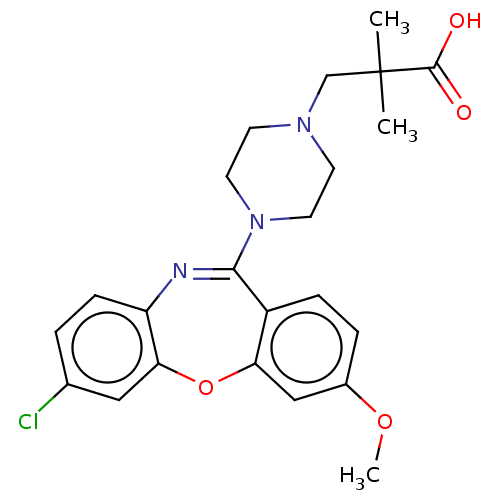 (US11046651, Compound 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 37.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM506780 (US11046651, Compound 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 37.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506783 (US11046651, Compound 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 37.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM506773 (US11046651, Compound 13) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 37.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM506786 (US11046651, Compound 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506786 (US11046651, Compound 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506791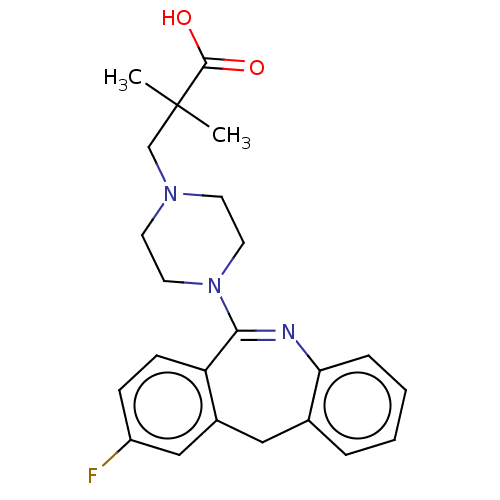 (US11046651, Compound 37) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506794 (US11046651, Compound 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506795 (US11046651, Compound 39) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM506779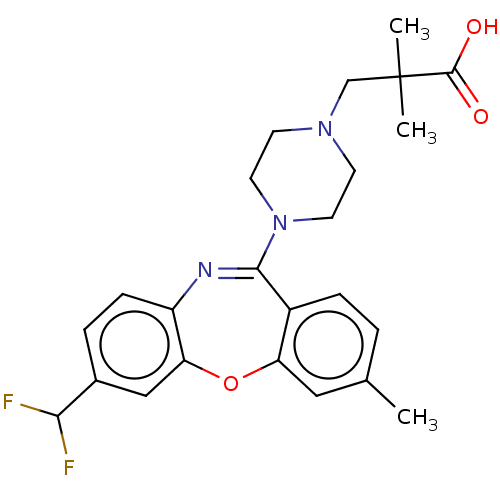 (US11046651, Compound 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM506795 (US11046651, Compound 39) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM506793 (US11046651, Compound 38) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM506792 (US11046651, Compound 11) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM506791 (US11046651, Compound 37) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM506789 (US11046651, Compound 21) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1 μl of a compounds of the present disclosure and either reference compound (Table C) were transferred to assay plates. 1 μl of 0.2 mM Keta... | Citation and Details BindingDB Entry DOI: 10.7270/Q20G3P9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 75 total ) | Next | Last >> |