

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM474223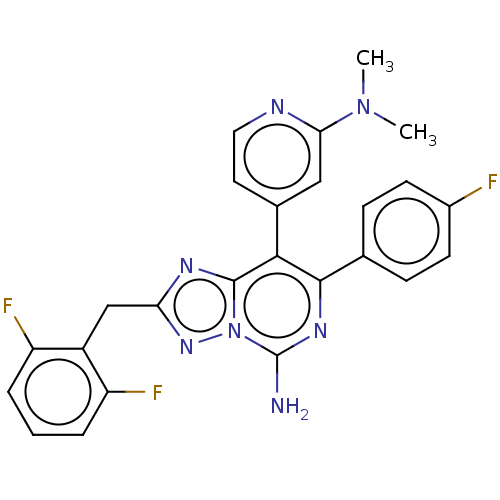 (2-(2,6-difluorobenzyl)-8-(2-(dimethylamino) pyridi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description The compounds at different concentrations were incubate with hA1 membrane (from PerkinElmer) and [3H]-8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) for ... | US Patent US10858365 (2020) BindingDB Entry DOI: 10.7270/Q2Q81H5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM474223 (2-(2,6-difluorobenzyl)-8-(2-(dimethylamino) pyridi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2W099V1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM474223 (2-(2,6-difluorobenzyl)-8-(2-(dimethylamino) pyridi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description hADORA1/CHO (hA1 expressing) cells (Genscript) were plated at 1×104 cells/well into 384-well polystyrene plates one day before starting the experimen... | US Patent US10858365 (2020) BindingDB Entry DOI: 10.7270/Q2Q81H5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||