

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50051653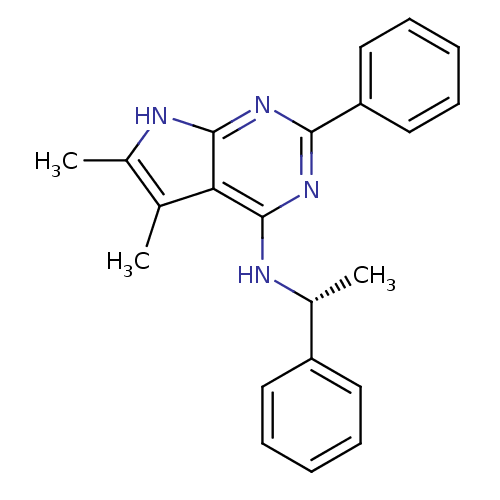 ((5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyrimidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leipzig Curated by ChEMBL | Assay Description Tested for its binding affinity at adenosine A1 receptor in rat brain cortical membrane preparations using [3H]-CCPA as a radioligand | J Med Chem 43: 4636-46 (2001) BindingDB Entry DOI: 10.7270/Q2SB450S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50051653 ((5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyrimidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universität Würzburg Curated by ChEMBL | Assay Description Radioligand binding assay for [3H]-PIA affinity towards Adenosine A1 receptor of rat cerebral cortical membrane | J Med Chem 39: 2482-91 (1996) Article DOI: 10.1021/jm960011w BindingDB Entry DOI: 10.7270/Q27H1HPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50051653 ((5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyrimidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity towards rat A1 receptor was determined | Bioorg Med Chem Lett 9: 2413-8 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50051653 ((5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyrimidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 981 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation Curated by ChEMBL | Assay Description Inhibitory activity against binding of [3H]-DPCPX to human A1 receptor (hA1) using competition binding assay | Bioorg Med Chem Lett 9: 2413-8 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50051653 ((5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyrimidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation Curated by ChEMBL | Assay Description Inhibitory activity against membranes from yeast cells transformed with human A1 receptor (hA1) | Bioorg Med Chem Lett 9: 2413-8 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50051653 ((5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyrimidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation Curated by ChEMBL | Assay Description Inhibitory activity against binding of [3H]-CGS-21,680 to human A2a receptor (hA2a) using competition binding assay | Bioorg Med Chem Lett 9: 2413-8 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||