

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A1 (BOVINE) | BDBM82013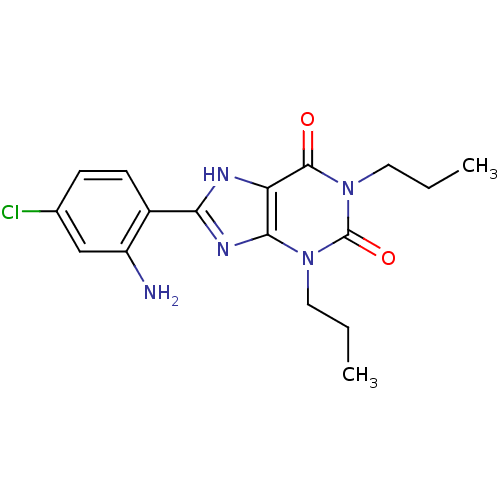 (8-(2-Amino-4-chloro-phenyl)-1,3-dipropyl-3,7-dihyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against adenosine A1 receptor in bovine brain membranes | J Med Chem 26: 1667-72 (1984) BindingDB Entry DOI: 10.7270/Q2H70DVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (BOVINE) | BDBM82013 (8-(2-Amino-4-chloro-phenyl)-1,3-dipropyl-3,7-dihyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by PDSP Ki Database | Naunyn Schmiedebergs Arch Pharmacol 330: 212-21 (1985) Article DOI: 10.1007/bf00572436 BindingDB Entry DOI: 10.7270/Q2RB7334 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM82013 (8-(2-Amino-4-chloro-phenyl)-1,3-dipropyl-3,7-dihyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity of the compound against A1 adenosine receptors of the central nervous system | J Med Chem 29: 1520-4 (1986) BindingDB Entry DOI: 10.7270/Q25Q4V25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM82013 (8-(2-Amino-4-chloro-phenyl)-1,3-dipropyl-3,7-dihyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonism of binding of 1 nM [3H]cyclohexyladenosine to adenosine A1 receptors on rat cortical membranes | J Med Chem 28: 487-92 (1985) BindingDB Entry DOI: 10.7270/Q2RV0P8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM82013 (8-(2-Amino-4-chloro-phenyl)-1,3-dipropyl-3,7-dihyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using [3H]-CHA or [3H]PIA as radioligand | J Med Chem 35: 407-22 (1992) BindingDB Entry DOI: 10.7270/Q2Z89D1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM82013 (8-(2-Amino-4-chloro-phenyl)-1,3-dipropyl-3,7-dihyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research Curated by PDSP Ki Database | Mol Pharmacol 29: 331-46 (1986) BindingDB Entry DOI: 10.7270/Q2MK6BDV | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM82013 (8-(2-Amino-4-chloro-phenyl)-1,3-dipropyl-3,7-dihyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Bio-Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of [3H]-CHA binding to Adenosine A1 receptor in rat brain membranes | J Med Chem 33: 1708-13 (1990) BindingDB Entry DOI: 10.7270/Q2CZ3643 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM82013 (8-(2-Amino-4-chloro-phenyl)-1,3-dipropyl-3,7-dihyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by PDSP Ki Database | Naunyn Schmiedebergs Arch Pharmacol 330: 212-21 (1985) Article DOI: 10.1007/bf00572436 BindingDB Entry DOI: 10.7270/Q2RB7334 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (BOVINE) | BDBM82013 (8-(2-Amino-4-chloro-phenyl)-1,3-dipropyl-3,7-dihyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against bovine brain adenosine A1 receptor by using N6-[3H]- cyclohexyladenosine | J Med Chem 28: 1071-9 (1985) Checked by Author BindingDB Entry DOI: 10.7270/Q2445N2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||