

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenylate cyclase type 10 (Homo sapiens (Human)) | BDBM492902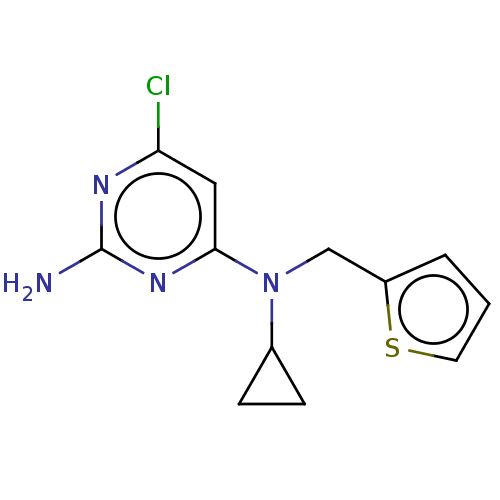 (US10981899, Example RU-0204277-LRE1) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00273 BindingDB Entry DOI: 10.7270/Q29S1VWQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenylate cyclase type 10 (Homo sapiens (Human)) | BDBM492902 (US10981899, Example RU-0204277-LRE1) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University; Tri-Institutional Therapeutics Discovery Institute US Patent | Assay Description The RapidFire 365 High-throughput MS System (Agilent Technologies; RF-MSS) can process samples every 15 seconds allowing analysis of a 384 well plate... | US Patent US10981899 (2021) BindingDB Entry DOI: 10.7270/Q2WD43Q0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenylate cyclase type 10 (Homo sapiens (Human)) | BDBM492902 (US10981899, Example RU-0204277-LRE1) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University; Tri-Institutional Therapeutics Discovery Institute US Patent | Assay Description INS-1E insulinoma cells were incubated in 2.5 mM glucose Krebs-Ringer buffer (pH 7.5) supplemented with 2 mM sodium bicarbonate, 10 mM HEPES, and 0.1... | US Patent US10981899 (2021) BindingDB Entry DOI: 10.7270/Q2WD43Q0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||