

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Homo sapiens (Human)) | BDBM50097373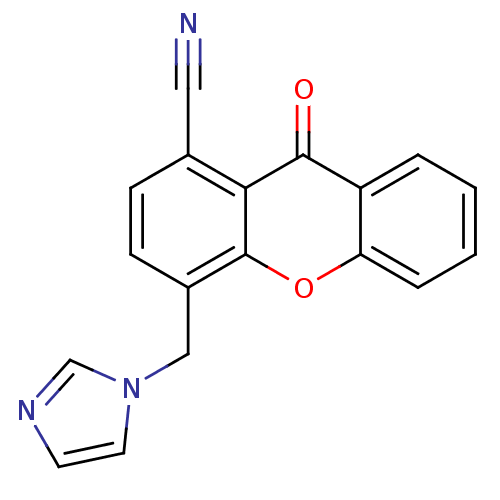 (4-((1H-imidazol-1-yl)methyl)-9-oxo-9H-xanthene-1-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human placental microsome cytochrome P450 19A1 aromatase | J Med Chem 44: 672-80 (2001) BindingDB Entry DOI: 10.7270/Q28S4P6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50097373 (4-((1H-imidazol-1-yl)methyl)-9-oxo-9H-xanthene-1-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of human aromatase using [1beta,2beta3H]-]testosterone as substrate | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50097373 (4-((1H-imidazol-1-yl)methyl)-9-oxo-9H-xanthene-1-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Coimbra Curated by ChEMBL | Assay Description Inhibition of human aromatase-mediated conversion of [1beta3H]androstenedione to estrone by liquid scintillation counting in presence of NADPH | J Med Chem 52: 143-50 (2009) Article DOI: 10.1021/jm800945c BindingDB Entry DOI: 10.7270/Q2SQ9080 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50097373 (4-((1H-imidazol-1-yl)methyl)-9-oxo-9H-xanthene-1-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah Curated by ChEMBL | Assay Description Inhibition of human placental aromatase in presence of [1beta,2beta-3H] testosterone by Thompson and Siiteri method | Eur J Med Chem 102: 375-86 (2015) Article DOI: 10.1016/j.ejmech.2015.08.010 BindingDB Entry DOI: 10.7270/Q2ZC84Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||