

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50540171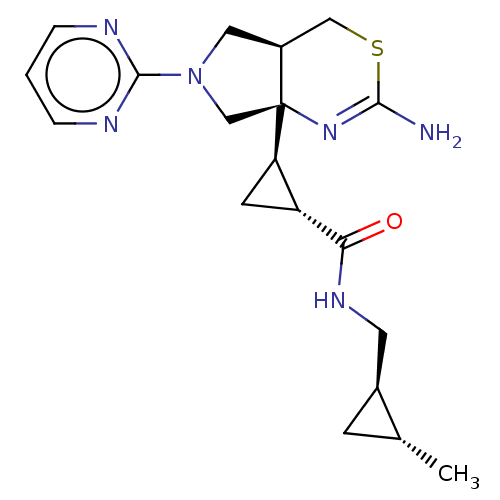 (CHEMBL4643727) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) by cell based assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115194 BindingDB Entry DOI: 10.7270/Q2B56P8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50540171 (CHEMBL4643727) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00489 BindingDB Entry DOI: 10.7270/Q29W0KBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50540171 (CHEMBL4643727) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) expressed in HEK293 cells using mcaFRET peptide as substrate after 20 hrs by FRET assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115194 BindingDB Entry DOI: 10.7270/Q2B56P8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||