

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50295541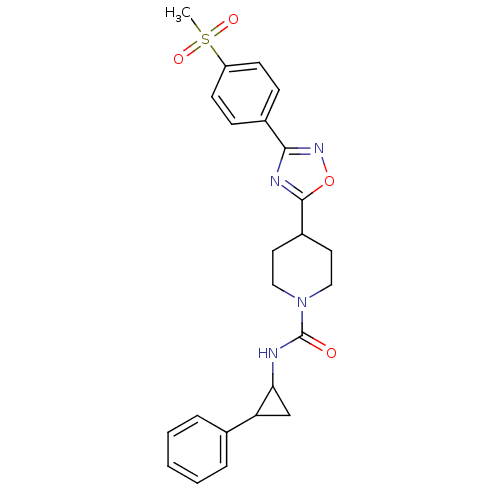 (4-(3-(4-(methylsulfonyl)phenyl)-1,2,4-oxadiazol-5-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human sEH | J Med Chem 52: 5009-12 (2010) Article DOI: 10.1021/jm900725r BindingDB Entry DOI: 10.7270/Q2TX3G8W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50295541 (4-(3-(4-(methylsulfonyl)phenyl)-1,2,4-oxadiazol-5-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human sEH in HEK293 cells assessed as conversion of 14,15-epoxyeicosatrienoic acid to 14,15-dihydroepoxyeicosatrienoic acid | J Med Chem 52: 5009-12 (2010) Article DOI: 10.1021/jm900725r BindingDB Entry DOI: 10.7270/Q2TX3G8W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Rattus norvegicus) | BDBM50295541 (4-(3-(4-(methylsulfonyl)phenyl)-1,2,4-oxadiazol-5-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat sEH | J Med Chem 52: 5009-12 (2010) Article DOI: 10.1021/jm900725r BindingDB Entry DOI: 10.7270/Q2TX3G8W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||