

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50006878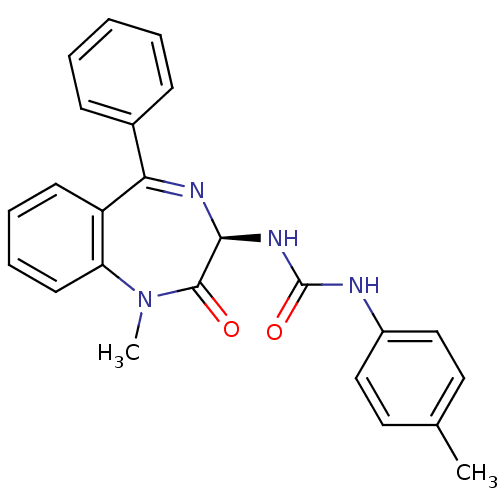 ((R)-1-(1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro binding affinity for the cholecystokinin type A receptor in guinea pig pancreas assayed using [125I]-BH-CCK-8 as radioligand | J Med Chem 37: 3789-811 (1994) BindingDB Entry DOI: 10.7270/Q2KK99TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50006878 ((R)-1-(1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding activity towards cholecystokinin-A (CCK-A) receptor in guinea pig pancreas | Bioorg Med Chem Lett 5: 1933-1936 (1995) Article DOI: 10.1016/0960-894X(95)00327-P BindingDB Entry DOI: 10.7270/Q22Z15H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50006878 ((R)-1-(1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against cholecystokinin-A (CCK-A) receptor in pancreas of guinea pig. | Bioorg Med Chem Lett 4: 2877-2882 (1994) Article DOI: 10.1016/S0960-894X(01)80832-5 BindingDB Entry DOI: 10.7270/Q24B318F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50006878 ((R)-1-(1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-Cholecystokinin-8 binding to Cholecystokinin type A receptor of rat pancreatic membranes | Bioorg Med Chem Lett 7: 169-174 (1997) Article DOI: 10.1016/S0960-894X(96)00609-9 BindingDB Entry DOI: 10.7270/Q27M07ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50006878 ((R)-1-(1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its activity to inhibit the binding of [125I]-CCK-8 to Cholecystokinin type A receptor in rat pancreas | Bioorg Med Chem Lett 3: 875-880 (1993) Article DOI: 10.1016/S0960-894X(00)80684-8 BindingDB Entry DOI: 10.7270/Q21C1WTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50006878 ((R)-1-(1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 736 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro test for inhibition of [125I]-CCK binding to Cholecystokinin type A receptor from rat pancreatic tissues was determined | Bioorg Med Chem Lett 3: 1919-1924 (1993) Article DOI: 10.1016/S0960-894X(01)80987-2 BindingDB Entry DOI: 10.7270/Q23J3CWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50006878 ((R)-1-(1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 736 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of ligand binding to Cholecystokinin type A receptor receptor from rat pancreatic tissue. | J Med Chem 40: 2491-501 (1997) Article DOI: 10.1021/jm9608523 BindingDB Entry DOI: 10.7270/Q2F47N84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50006878 ((R)-1-(1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 736 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]-BH CCK-8S from Cholecystokinin type A receptor in rat pancreas | Bioorg Med Chem Lett 5: 3023-3026 (1995) Article DOI: 10.1016/0960-894X(95)00530-0 BindingDB Entry DOI: 10.7270/Q2VX0GGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50006878 ((R)-1-(1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 736 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience Research Centre Curated by ChEMBL | Assay Description Inhibition of [125 I]BH CCK-8S binding to Cholecystokinin type A receptor in pancreatic tissue | J Med Chem 39: 842-9 (1996) Article DOI: 10.1021/jm9506736 BindingDB Entry DOI: 10.7270/Q25T3JJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50006878 ((R)-1-(1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type A receptor (CCK-A) receptor from rat pancreas using [125I]-Bolton Hunter CCK-8 as radioligand | J Med Chem 41: 1042-9 (1998) Article DOI: 10.1021/jm970373j BindingDB Entry DOI: 10.7270/Q2TQ60N3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50006878 ((R)-1-(1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [125I]CCK-8 to cholecystokinin type A receptor in rat pancreatic tissue | J Med Chem 36: 4276-92 (1994) BindingDB Entry DOI: 10.7270/Q22J6CH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Mus musculus) | BDBM50006878 ((R)-1-(1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Cholecystokinin type A receptor | Bioorg Med Chem Lett 6: 1421-1426 (1996) Article DOI: 10.1016/S0960-894X(96)00248-X BindingDB Entry DOI: 10.7270/Q2251J56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Mus musculus) | BDBM50006878 ((R)-1-(1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Cholecystokinin type A receptor | Bioorg Med Chem Lett 6: 1427-1430 (1996) Article DOI: 10.1016/S0960-894X(96)00249-1 BindingDB Entry DOI: 10.7270/Q2XD11NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||