

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyclin-G-associated kinase (Homo sapiens (Human)) | BDBM50290836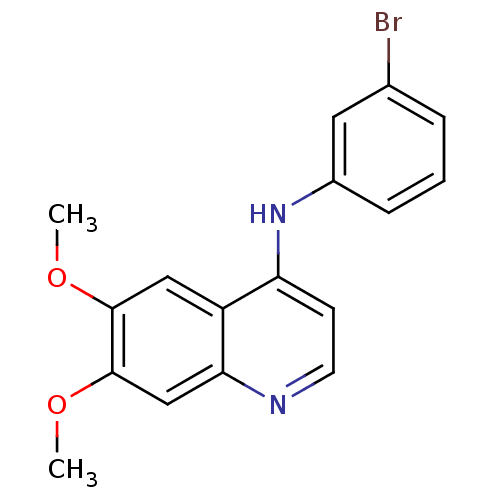 ((3-Bromo-phenyl)-(6,7-dimethoxy-quinolin-4-yl)-ami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of tracer 5 binding to human N-terminal nano luciferase-fused GAK expressed in HEK293 cells measured after 2 hrs by nanoBRET assay | J Med Chem 62: 4772-4778 (2019) Article DOI: 10.1021/acs.jmedchem.9b00350 BindingDB Entry DOI: 10.7270/Q2RJ4NVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-G-associated kinase (Homo sapiens (Human)) | BDBM50290836 ((3-Bromo-phenyl)-(6,7-dimethoxy-quinolin-4-yl)-ami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Binding affinity to wild-type human partial length GAK (G13 to Y338 residues) expressed in bacterial expression system by Kinomescan method | J Med Chem 62: 4772-4778 (2019) Article DOI: 10.1021/acs.jmedchem.9b00350 BindingDB Entry DOI: 10.7270/Q2RJ4NVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||