

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25308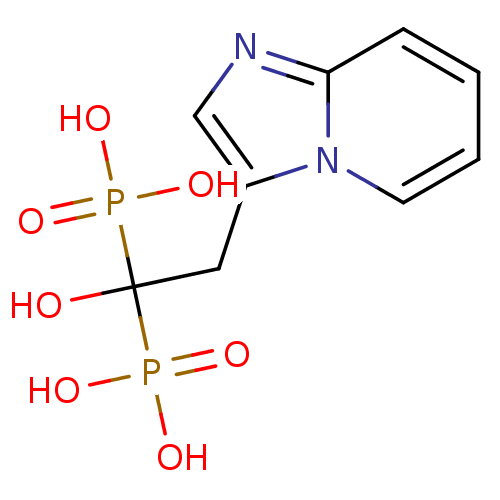 ((1-hydroxy-2-{imidazo[1,2-a]pyridin-3-yl}-1-phosph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry Curated by ChEMBL | Assay Description Binding affinity to human GGPPS | Proc Natl Acad Sci USA 104: 10022-7 (2007) Article DOI: 10.1073/pnas.0702254104 BindingDB Entry DOI: 10.7270/Q2MW2J2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25308 ((1-hydroxy-2-{imidazo[1,2-a]pyridin-3-yl}-1-phosph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibition of GGPPS after 10 mins using [14C]IPP as substrate by liquid scintillation counting | J Med Chem 53: 3454-64 (2010) Checked by Author Article DOI: 10.1021/jm900232u BindingDB Entry DOI: 10.7270/Q21837FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25308 ((1-hydroxy-2-{imidazo[1,2-a]pyridin-3-yl}-1-phosph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry Curated by ChEMBL | Assay Description Inhibition of human GGPPS | Proc Natl Acad Sci USA 104: 10022-7 (2007) Article DOI: 10.1073/pnas.0702254104 BindingDB Entry DOI: 10.7270/Q2MW2J2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25308 ((1-hydroxy-2-{imidazo[1,2-a]pyridin-3-yl}-1-phosph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.07E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||