 Found 10 hits Enz. Inhib. hit(s) with Target = 'Glutamate receptor ionotropic, kainate 1' and Ligand = 'BDBM50002369'
Found 10 hits Enz. Inhib. hit(s) with Target = 'Glutamate receptor ionotropic, kainate 1' and Ligand = 'BDBM50002369' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate receptor ionotropic, kainate 1
(RAT) | BDBM50002369
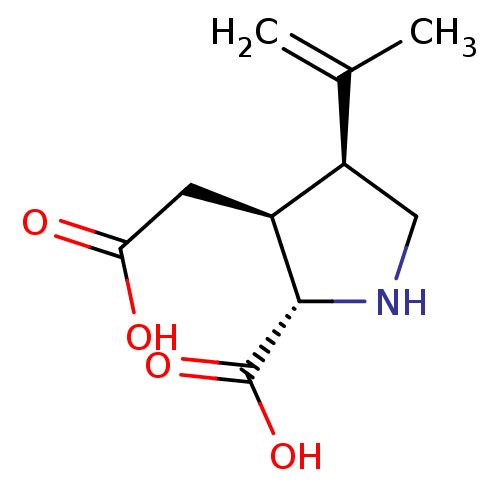
((2S-(2alpha,3beta,4beta))-2-carboxy-4-(1-methyleth...)Show SMILES CC(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O |r| Show InChI InChI=1S/C10H15NO4/c1-5(2)7-4-11-9(10(14)15)6(7)3-8(12)13/h6-7,9,11H,1,3-4H2,2H3,(H,12,13)(H,14,15)/t6-,7+,9-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Institute for Bioorganic Research
Curated by PDSP Ki Database
| |
Mol Pharmacol 53: 195-201 (1998)
Article DOI: 10.1124/mol.53.2.195
BindingDB Entry DOI: 10.7270/Q2RF5SJH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, kainate 1
(RAT) | BDBM50002369

((2S-(2alpha,3beta,4beta))-2-carboxy-4-(1-methyleth...)Show SMILES CC(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O |r| Show InChI InChI=1S/C10H15NO4/c1-5(2)7-4-11-9(10(14)15)6(7)3-8(12)13/h6-7,9,11H,1,3-4H2,2H3,(H,12,13)(H,14,15)/t6-,7+,9-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 75.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Blaise Pascal
Curated by ChEMBL
| Assay Description
Displacement of [3H]SYM2081 from rat recombinant iGluR5 |
J Med Chem 51: 4093-103 (2008)
Article DOI: 10.1021/jm800092x
BindingDB Entry DOI: 10.7270/Q20V8DP6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, kainate 1
(RAT) | BDBM50002369

((2S-(2alpha,3beta,4beta))-2-carboxy-4-(1-methyleth...)Show SMILES CC(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O |r| Show InChI InChI=1S/C10H15NO4/c1-5(2)7-4-11-9(10(14)15)6(7)3-8(12)13/h6-7,9,11H,1,3-4H2,2H3,(H,12,13)(H,14,15)/t6-,7+,9-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
European Research Centre for Drug Discovery and Development (NatSynDrugs)
Curated by ChEMBL
| Assay Description
Displacement of [3H]SYM2081 from rat recombinant iGluR5(Q)1b expressed in Sf9 cells |
J Med Chem 51: 6614-8 (2008)
Article DOI: 10.1021/jm800865a
BindingDB Entry DOI: 10.7270/Q261117R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50002369

((2S-(2alpha,3beta,4beta))-2-carboxy-4-(1-methyleth...)Show SMILES CC(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O |r| Show InChI InChI=1S/C10H15NO4/c1-5(2)7-4-11-9(10(14)15)6(7)3-8(12)13/h6-7,9,11H,1,3-4H2,2H3,(H,12,13)(H,14,15)/t6-,7+,9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 177 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly, S.A.
Curated by ChEMBL
| Assay Description
Binding affinity against human ionotropic glutamate receptor kainate 1 in HK293 cells using [3H]-kainate as radioligand |
J Med Chem 43: 1958-68 (2000)
BindingDB Entry DOI: 10.7270/Q2FX78QB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50002369

((2S-(2alpha,3beta,4beta))-2-carboxy-4-(1-methyleth...)Show SMILES CC(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O |r| Show InChI InChI=1S/C10H15NO4/c1-5(2)7-4-11-9(10(14)15)6(7)3-8(12)13/h6-7,9,11H,1,3-4H2,2H3,(H,12,13)(H,14,15)/t6-,7+,9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 177 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Ltd
Curated by ChEMBL
| Assay Description
Binding affinity of compound was determined against Ionotropic glutamate receptor ionotropic kainate 1 using cell membranes prepared from HEK293 cell... |
Bioorg Med Chem Lett 10: 1807-10 (2000)
BindingDB Entry DOI: 10.7270/Q2765FVB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, kainate 1
(RAT) | BDBM50002369

((2S-(2alpha,3beta,4beta))-2-carboxy-4-(1-methyleth...)Show SMILES CC(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O |r| Show InChI InChI=1S/C10H15NO4/c1-5(2)7-4-11-9(10(14)15)6(7)3-8(12)13/h6-7,9,11H,1,3-4H2,2H3,(H,12,13)(H,14,15)/t6-,7+,9-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Carlo Bo
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Adenosine A3 receptor expressed in HEK293 cells using 0.1 nM [3H]AB-MECA |
J Med Chem 48: 6887-96 (2005)
Article DOI: 10.1021/jm058018d
BindingDB Entry DOI: 10.7270/Q28C9X1X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, kainate 1
(RAT) | BDBM50002369

((2S-(2alpha,3beta,4beta))-2-carboxy-4-(1-methyleth...)Show SMILES CC(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O |r| Show InChI InChI=1S/C10H15NO4/c1-5(2)7-4-11-9(10(14)15)6(7)3-8(12)13/h6-7,9,11H,1,3-4H2,2H3,(H,12,13)(H,14,15)/t6-,7+,9-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Agonist activity at rat recombinant GluR5(Q) RNA-edited isoform expressed in HEK293 cells assessed as increase in intracellular calcium level by Fluo... |
J Med Chem 52: 4911-22 (2009)
Checked by Author
Article DOI: 10.1021/jm900565c
BindingDB Entry DOI: 10.7270/Q2R49RP9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50002369

((2S-(2alpha,3beta,4beta))-2-carboxy-4-(1-methyleth...)Show SMILES CC(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O |r| Show InChI InChI=1S/C10H15NO4/c1-5(2)7-4-11-9(10(14)15)6(7)3-8(12)13/h6-7,9,11H,1,3-4H2,2H3,(H,12,13)(H,14,15)/t6-,7+,9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | n/a | n/a | 1.62E+4 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Ltd
Curated by ChEMBL
| Assay Description
Compound was tested for agonistic activity at Glutamate receptor 5 using HEK293 cells |
Bioorg Med Chem Lett 10: 1807-10 (2000)
BindingDB Entry DOI: 10.7270/Q2765FVB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50002369

((2S-(2alpha,3beta,4beta))-2-carboxy-4-(1-methyleth...)Show SMILES CC(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O |r| Show InChI InChI=1S/C10H15NO4/c1-5(2)7-4-11-9(10(14)15)6(7)3-8(12)13/h6-7,9,11H,1,3-4H2,2H3,(H,12,13)(H,14,15)/t6-,7+,9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | n/a | n/a | 1.62E+4 | n/a | n/a | n/a | n/a |
Lilly, S.A.
Curated by ChEMBL
| Assay Description
Effective concentration against GluR5 expressed in HEK293 cells |
J Med Chem 43: 1958-68 (2000)
BindingDB Entry DOI: 10.7270/Q2FX78QB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, kainate 1
(RAT) | BDBM50002369

((2S-(2alpha,3beta,4beta))-2-carboxy-4-(1-methyleth...)Show SMILES CC(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O |r| Show InChI InChI=1S/C10H15NO4/c1-5(2)7-4-11-9(10(14)15)6(7)3-8(12)13/h6-7,9,11H,1,3-4H2,2H3,(H,12,13)(H,14,15)/t6-,7+,9-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Ltd
Curated by ChEMBL
| Assay Description
Compound was tested for agonistic activity on rat dorsal root ganglion neurons (thought to express GluR5 receptors) |
Bioorg Med Chem Lett 10: 1807-10 (2000)
BindingDB Entry DOI: 10.7270/Q2765FVB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data