 Found 8 hits Enz. Inhib. hit(s) with Target = 'Histone acetyltransferase p300' and Ligand = 'BDBM50151658'
Found 8 hits Enz. Inhib. hit(s) with Target = 'Histone acetyltransferase p300' and Ligand = 'BDBM50151658' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50151658
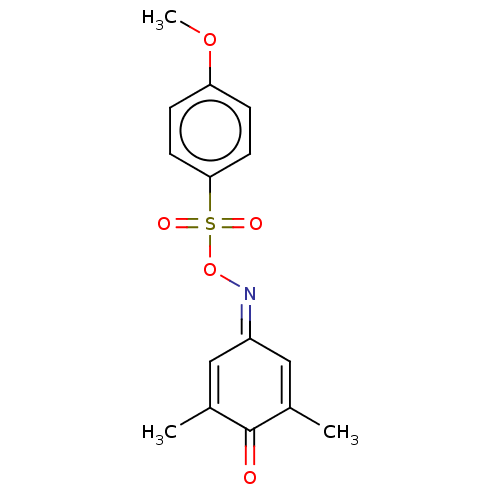
(CHEMBL3775671)Show SMILES [#6]-[#8]-c1ccc(cc1)S(=O)(=O)[#8]\[#7]=[#6]-1/[#6]=[#6](-[#6])-[#6](=O)-[#6](-[#6])=[#6]-1 |c:21,t:15| Show InChI InChI=1S/C15H15NO5S/c1-10-8-12(9-11(2)15(10)17)16-21-22(18,19)14-6-4-13(20-3)5-7-14/h4-9H,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of P300 (unknown origin) using histone H3 as substrate by fluorescence assay |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50151658

(CHEMBL3775671)Show SMILES [#6]-[#8]-c1ccc(cc1)S(=O)(=O)[#8]\[#7]=[#6]-1/[#6]=[#6](-[#6])-[#6](=O)-[#6](-[#6])=[#6]-1 |c:21,t:15| Show InChI InChI=1S/C15H15NO5S/c1-10-8-12(9-11(2)15(10)17)16-21-22(18,19)14-6-4-13(20-3)5-7-14/h4-9H,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of P300 catalytic domain (unknown origin) using histone H3 and [acetyl-3H]-acetylcoenzyme A as substrate preincubated for 15 mins followed... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112861
BindingDB Entry DOI: 10.7270/Q2CC14D7 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50151658

(CHEMBL3775671)Show SMILES [#6]-[#8]-c1ccc(cc1)S(=O)(=O)[#8]\[#7]=[#6]-1/[#6]=[#6](-[#6])-[#6](=O)-[#6](-[#6])=[#6]-1 |c:21,t:15| Show InChI InChI=1S/C15H15NO5S/c1-10-8-12(9-11(2)15(10)17)16-21-22(18,19)14-6-4-13(20-3)5-7-14/h4-9H,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by ChEMBL
| Assay Description
Inhibition of p300 catalytic domain (unknown origin) using histone H3 as substrate preincubated for 15 mins followed by substrate addition measured a... |
J Med Chem 61: 3239-3252 (2018)
Article DOI: 10.1021/acs.jmedchem.6b01817
BindingDB Entry DOI: 10.7270/Q2542R5N |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50151658

(CHEMBL3775671)Show SMILES [#6]-[#8]-c1ccc(cc1)S(=O)(=O)[#8]\[#7]=[#6]-1/[#6]=[#6](-[#6])-[#6](=O)-[#6](-[#6])=[#6]-1 |c:21,t:15| Show InChI InChI=1S/C15H15NO5S/c1-10-8-12(9-11(2)15(10)17)16-21-22(18,19)14-6-4-13(20-3)5-7-14/h4-9H,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg
Curated by ChEMBL
| Assay Description
Inhibition of recombinant p300 catalytic domain (unknown origin) using N-terminal histone H3 substrate by orthogonal fluorescence assay in presence o... |
J Med Chem 59: 1249-70 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01502
BindingDB Entry DOI: 10.7270/Q2DV1MRN |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50151658

(CHEMBL3775671)Show SMILES [#6]-[#8]-c1ccc(cc1)S(=O)(=O)[#8]\[#7]=[#6]-1/[#6]=[#6](-[#6])-[#6](=O)-[#6](-[#6])=[#6]-1 |c:21,t:15| Show InChI InChI=1S/C15H15NO5S/c1-10-8-12(9-11(2)15(10)17)16-21-22(18,19)14-6-4-13(20-3)5-7-14/h4-9H,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114407
BindingDB Entry DOI: 10.7270/Q2MP578G |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50151658

(CHEMBL3775671)Show SMILES [#6]-[#8]-c1ccc(cc1)S(=O)(=O)[#8]\[#7]=[#6]-1/[#6]=[#6](-[#6])-[#6](=O)-[#6](-[#6])=[#6]-1 |c:21,t:15| Show InChI InChI=1S/C15H15NO5S/c1-10-8-12(9-11(2)15(10)17)16-21-22(18,19)14-6-4-13(20-3)5-7-14/h4-9H,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Avera Institute for Human Genetics
Curated by ChEMBL
| Assay Description
Inhibition of recombinant p300 catalytic domain (unknown origin) using histone H3 N-terminal peptide as substrate in presence of acetyl CoA by fluore... |
J Med Chem 63: 4716-4731 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02164
BindingDB Entry DOI: 10.7270/Q27P92QB |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50151658

(CHEMBL3775671)Show SMILES [#6]-[#8]-c1ccc(cc1)S(=O)(=O)[#8]\[#7]=[#6]-1/[#6]=[#6](-[#6])-[#6](=O)-[#6](-[#6])=[#6]-1 |c:21,t:15| Show InChI InChI=1S/C15H15NO5S/c1-10-8-12(9-11(2)15(10)17)16-21-22(18,19)14-6-4-13(20-3)5-7-14/h4-9H,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of P300 (unknown origin) |
Eur J Med Chem 178: 259-286 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.078
BindingDB Entry DOI: 10.7270/Q2ZW1QC8 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50151658

(CHEMBL3775671)Show SMILES [#6]-[#8]-c1ccc(cc1)S(=O)(=O)[#8]\[#7]=[#6]-1/[#6]=[#6](-[#6])-[#6](=O)-[#6](-[#6])=[#6]-1 |c:21,t:15| Show InChI InChI=1S/C15H15NO5S/c1-10-8-12(9-11(2)15(10)17)16-21-22(18,19)14-6-4-13(20-3)5-7-14/h4-9H,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.28E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg
Curated by ChEMBL
| Assay Description
Inhibition of recombinant p300 catalytic domain (unknown origin) using N-terminal histone H3 substrate by radiometric filter binding based phenotypic... |
J Med Chem 59: 1249-70 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01502
BindingDB Entry DOI: 10.7270/Q2DV1MRN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data