

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50369345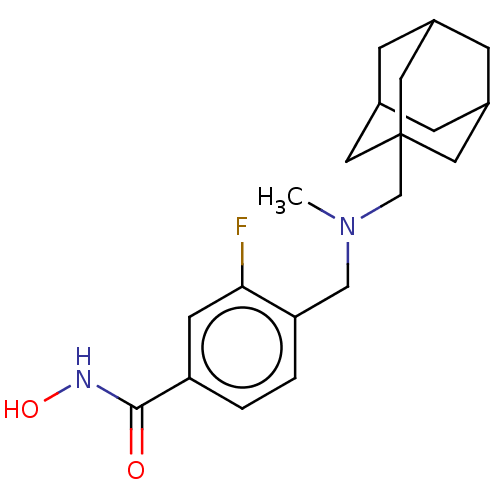 (CHEMBL4165724 | US11207431, Example 1 | US11890356...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description IC50 measurements were conducted by BPS Biosciences (Table 1) or by Nanosyn (Table 1A) with an established fluorescence assay. | Citation and Details BindingDB Entry DOI: 10.7270/Q2639SXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50369345 (CHEMBL4165724 | US11207431, Example 1 | US11890356...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using fluorogenic HDAC substrate by fluorescence assay | J Med Chem 62: 8557-8577 (2019) Article DOI: 10.1021/acs.jmedchem.9b00946 BindingDB Entry DOI: 10.7270/Q2542RXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50369345 (CHEMBL4165724 | US11207431, Example 1 | US11890356...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description IC50 measurements were conducted by BPS Biosciences (Table 1) or by Nanosyn (Table 1A) with an established fluorescence assay. | Citation and Details BindingDB Entry DOI: 10.7270/Q2639SXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50369345 (CHEMBL4165724 | US11207431, Example 1 | US11890356...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114090 BindingDB Entry DOI: 10.7270/Q2FF3XCC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50369345 (CHEMBL4165724 | US11207431, Example 1 | US11890356...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 incubated for 90 mins using fluorogenic substrate ZMAL (Z-Lys(Ac)-AMC) by fluorescence based assay | J Med Chem 61: 8054-8060 (2018) Article DOI: 10.1021/acs.jmedchem.8b01013 BindingDB Entry DOI: 10.7270/Q2KP84QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50369345 (CHEMBL4165724 | US11207431, Example 1 | US11890356...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against recombinant Trypanosoma cruzi (Trypanosoma cruzi) Trypanothione reductase | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||