

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM249366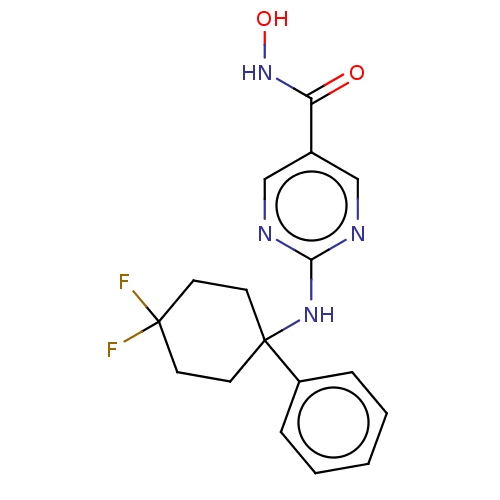 (US9464073, 003 | US9884850, Compound 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) using acetyl-lysine tripeptide as substrate preincubated for 24 hrs followed by substrate addition and measured ... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127023 BindingDB Entry DOI: 10.7270/Q23T9MS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM249366 (US9464073, 003 | US9884850, Compound 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HDAC6 (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00567 BindingDB Entry DOI: 10.7270/Q2JH3QW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM249366 (US9464073, 003 | US9884850, Compound 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114090 BindingDB Entry DOI: 10.7270/Q2FF3XCC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM249366 (US9464073, 003 | US9884850, Compound 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ACETYLON PHARMACEUTICALS, INC. US Patent | Assay Description Compounds for testing were diluted in DMSO to 50 fold the final concentration and a ten point three fold dilution series was made. The compounds were... | US Patent US9884850 (2018) BindingDB Entry DOI: 10.7270/Q2CZ3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM249366 (US9464073, 003 | US9884850, Compound 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Acetylon Pharmaceuticals, Inc. US Patent | Assay Description Compounds for testing were diluted in DMSO to 50 fold the final concentration and a ten point three fold dilution series was made. The compounds were... | US Patent US9464073 (2016) BindingDB Entry DOI: 10.7270/Q2TD9W8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||