

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM58106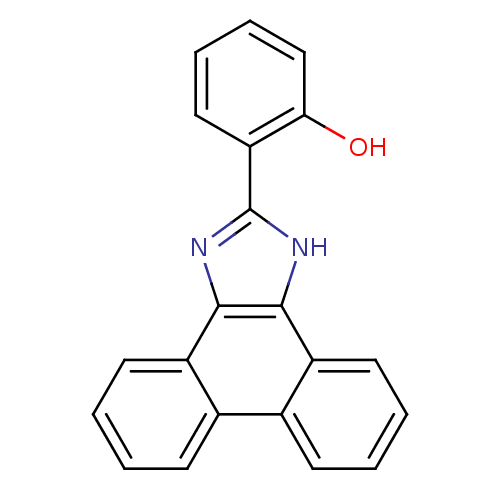 (6-(1,3-dihydrophenanthr[9,10-d]imidazol-2-ylidene)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 expressed in bacterial expression system using L-Tryptophan as substrate after 25 mins in absence of GSH and pre... | Eur J Med Chem 126: 983-996 (2017) Article DOI: 10.1016/j.ejmech.2016.12.029 BindingDB Entry DOI: 10.7270/Q2RX9F9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM58106 (6-(1,3-dihydrophenanthr[9,10-d]imidazol-2-ylidene)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 expressed in bacterial expression system using L-Tryptophan as substrate after 25 mins in presence of GSH and tw... | Eur J Med Chem 126: 983-996 (2017) Article DOI: 10.1016/j.ejmech.2016.12.029 BindingDB Entry DOI: 10.7270/Q2RX9F9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM58106 (6-(1,3-dihydrophenanthr[9,10-d]imidazol-2-ylidene)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Compound was tested in a cell-free SLe-polyacrylamide glycoconjugate binding assay (assay B) in P-selectin | Eur J Med Chem 126: 983-996 (2017) Article DOI: 10.1016/j.ejmech.2016.12.029 BindingDB Entry DOI: 10.7270/Q2RX9F9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM58106 (6-(1,3-dihydrophenanthr[9,10-d]imidazol-2-ylidene)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM58106 (6-(1,3-dihydrophenanthr[9,10-d]imidazol-2-ylidene)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Antagonistic activity against labelled Bombesin receptor bb2 binding sites in rat cerebral cortex by using [125I]- [Tyr] bombesin in presence of NMB;... | Eur J Med Chem 126: 983-996 (2017) Article DOI: 10.1016/j.ejmech.2016.12.029 BindingDB Entry DOI: 10.7270/Q2RX9F9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||