

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM473868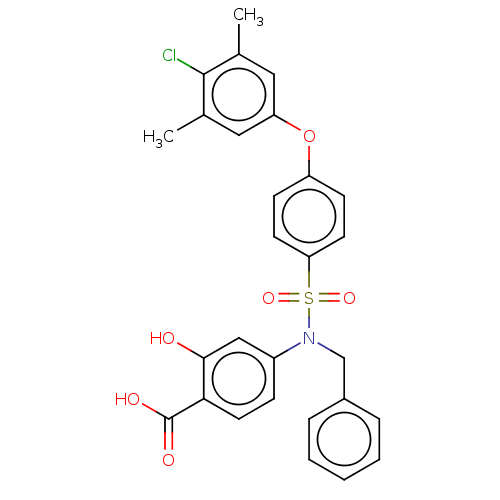 (4-(N-benzyl-4-(4- chloro-3,5- dimethylphenoxy) phe...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 629 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF MARYLAND, BALTIMORE US Patent | Assay Description Molecular modeling and SILCS functional group affinity mapping (FragMaps) of the Mcl-1 binding site indicated that the carboxylic acid of designed mo... | US Patent US10858316 (2020) BindingDB Entry DOI: 10.7270/Q20R9SHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM473868 (4-(N-benzyl-4-(4- chloro-3,5- dimethylphenoxy) phe...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 629 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM473868 (4-(N-benzyl-4-(4- chloro-3,5- dimethylphenoxy) phe...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 629 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF MARYLAND, BALTIMORE US Patent | Assay Description Molecular modeling and SILCS functional group affinity mapping (FragMaps) of the Mcl-1 binding site indicated that the carboxylic acid of designed mo... | US Patent US10858316 (2020) BindingDB Entry DOI: 10.7270/Q20R9SHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM473868 (4-(N-benzyl-4-(4- chloro-3,5- dimethylphenoxy) phe...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 659 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF MARYLAND, BALTIMORE US Patent | Assay Description Molecular modeling and SILCS functional group affinity mapping (FragMaps) of the Mcl-1 binding site indicated that the carboxylic acid of designed mo... | US Patent US10858316 (2020) BindingDB Entry DOI: 10.7270/Q20R9SHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||