

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403029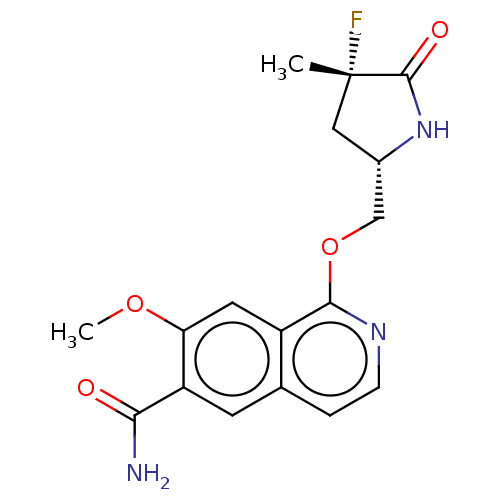 (US10329302, Example 177 | US10793579, Example 194 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... | US Patent US10793579 (2020) BindingDB Entry DOI: 10.7270/Q2H9988P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403029 (US10329302, Example 177 | US10793579, Example 194 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27D308N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403029 (US10329302, Example 177 | US10793579, Example 194 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description This is an in vitro assay to measure IRAK4 enzymatic activity utilizing the DELFIA (Dissociation-Enhanced Lanthanide Fluorescent Immunoassay, Perkin-... | ACS Chem Biol 4: 834-43 (2009) BindingDB Entry DOI: 10.7270/Q2FT8PC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM403029 (US10329302, Example 177 | US10793579, Example 194 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... | US Patent US10793579 (2020) BindingDB Entry DOI: 10.7270/Q2H9988P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||