

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysosomal Pro-X carboxypeptidase (Mus musculus) | BDBM50344263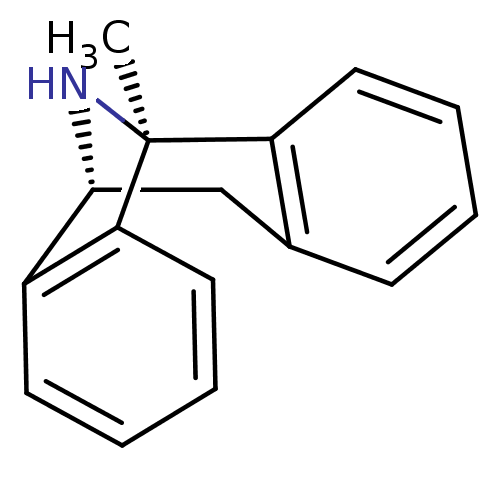 ((+)-1-methyl-16-azatetracyclo[7.6.1.02,7.010,15]he...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Carlo Bo Curated by ChEMBL | Assay Description Inhibitory concentration against human Adenosine A3 receptor expressed in HEK293 cells using 0.1 nM [3H]AB-MECA | J Med Chem 48: 6887-96 (2005) Article DOI: 10.1021/jm058018d BindingDB Entry DOI: 10.7270/Q28C9X1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal Pro-X carboxypeptidase (Homo sapiens (Human)) | BDBM50344263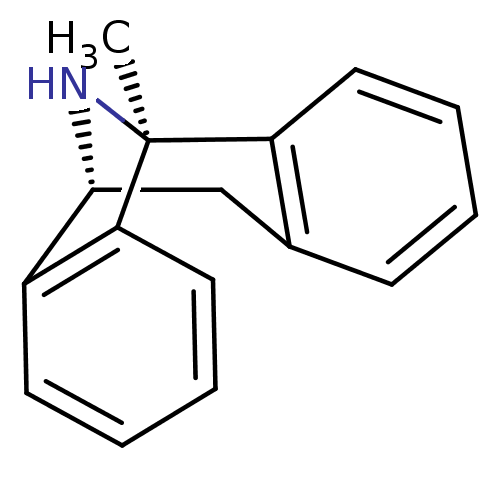 ((+)-1-methyl-16-azatetracyclo[7.6.1.02,7.010,15]he...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle& Co. Curated by ChEMBL | Assay Description Inhibition of [3H]1-[1-(2-thienyl) piperidine ([3H]TCP) binding to phencyclidine receptor | J Med Chem 32: 1242-8 (1989) BindingDB Entry DOI: 10.7270/Q2CR5TXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal Pro-X carboxypeptidase (Homo sapiens (Human)) | BDBM50344263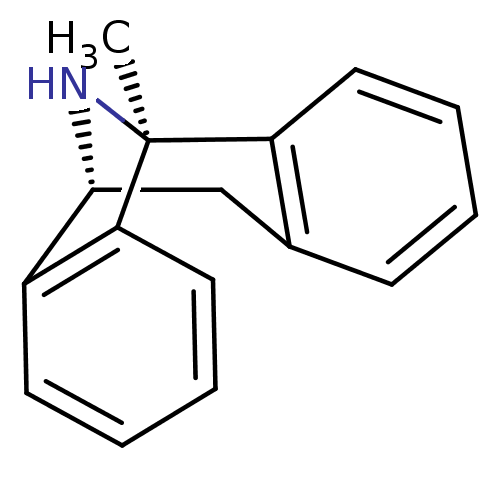 ((+)-1-methyl-16-azatetracyclo[7.6.1.02,7.010,15]he...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago 60607-7061 Curated by ChEMBL | Assay Description Inhibition of [3H]-TCP binding to PCP receptor obtained from tissue homogenate preparation of fresh whole rat brain minus cerebellum | J Med Chem 41: 468-77 (1998) Article DOI: 10.1021/jm970059p BindingDB Entry DOI: 10.7270/Q27W6CW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||