

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50428080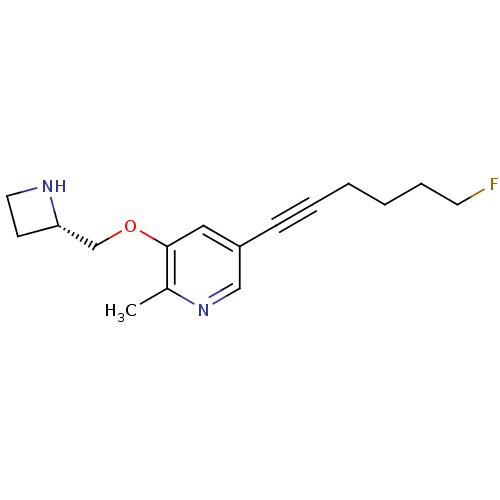 (CHEMBL2323571 | US9303017, (S)-22, YL-1-231) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 4.20 | -44.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Georgetown University; Duke University US Patent | Assay Description Briefly, cultured cells at >80% confluence were removed from their flasks (80 cm^2) with a disposable cell scraper and placed in 10 mL of 50 mM Tris.... | US Patent US9303017 (2016) BindingDB Entry DOI: 10.7270/Q25X27SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50428080 (CHEMBL2323571 | US9303017, (S)-22, YL-1-231) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 5.10 | -44.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Georgetown University; Duke University US Patent | Assay Description Briefly, cultured cells at >80% confluence were removed from their flasks (80 cm^2) with a disposable cell scraper and placed in 10 mL of 50 mM Tris.... | US Patent US9303017 (2016) BindingDB Entry DOI: 10.7270/Q25X27SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50428080 (CHEMBL2323571 | US9303017, (S)-22, YL-1-231) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University; Duke University US Patent | Assay Description IC50(10′): The functional properties of the ligands were determined by 86Rb+ efflux assays in cells expressing α3β4 and α4β... | US Patent US9303017 (2016) BindingDB Entry DOI: 10.7270/Q25X27SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50428080 (CHEMBL2323571 | US9303017, (S)-22, YL-1-231) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Desensitization of human alpha4beta2 nACHR expressed in HEK293 cells assessed as inhibition of 86Rb+ efflux preincubated for 10 mins measured after 2... | J Med Chem 56: 3000-11 (2013) Article DOI: 10.1021/jm4000374 BindingDB Entry DOI: 10.7270/Q21Z45R1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50428080 (CHEMBL2323571 | US9303017, (S)-22, YL-1-231) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University; Duke University US Patent | Assay Description IC50(10′): The functional properties of the ligands were determined by 86Rb+ efflux assays in cells expressing α3β4 and α4β... | US Patent US9303017 (2016) BindingDB Entry DOI: 10.7270/Q25X27SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||