

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50180872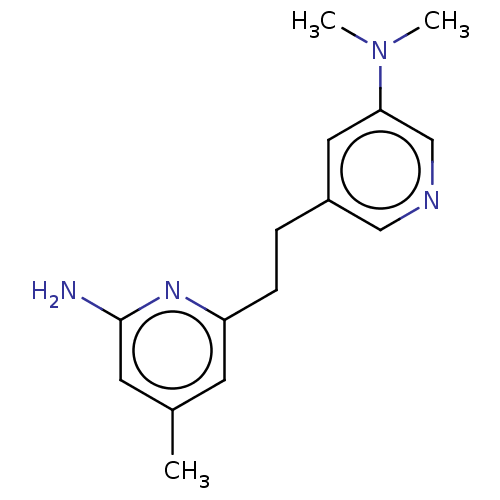 (CHEMBL3819046 | US10759791, Compound 14a) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 139 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Enzyme inhibition was evaluated by measuring NO production with the hemoglobin capture assay, which was performed with purified NOSs in 96-well plate... | US Patent US10759791 (2020) BindingDB Entry DOI: 10.7270/Q2ZW1PZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50180872 (CHEMBL3819046 | US10759791, Compound 14a) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 139 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of rat recombinant nNOS expressed in Escherichia coli using L-arginine as substrate assessed as reduction in NO production measured for 6 ... | J Med Chem 59: 4913-25 (2016) Article DOI: 10.1021/acs.jmedchem.6b00273 BindingDB Entry DOI: 10.7270/Q2J38VHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50180872 (CHEMBL3819046 | US10759791, Compound 14a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 425 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Enzyme inhibition was evaluated by measuring NO production with the hemoglobin capture assay, which was performed with purified NOSs in 96-well plate... | US Patent US10759791 (2020) BindingDB Entry DOI: 10.7270/Q2ZW1PZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50180872 (CHEMBL3819046 | US10759791, Compound 14a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 425 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Enzyme inhibition was evaluated by measuring NO production with the hemoglobin capture assay, which was performed with purified NOSs in 96-well plate... | US Patent US10759791 (2020) BindingDB Entry DOI: 10.7270/Q2ZW1PZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50180872 (CHEMBL3819046 | US10759791, Compound 14a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 425 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of human recombinant nNOS expressed in Escherichia coli using L-arginine as substrate assessed as reduction in NO production measured for ... | J Med Chem 59: 4913-25 (2016) Article DOI: 10.1021/acs.jmedchem.6b00273 BindingDB Entry DOI: 10.7270/Q2J38VHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||