 Found 4 hits Enz. Inhib. hit(s) with Target = 'Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform' and Ligand = 'BDBM50427453'
Found 4 hits Enz. Inhib. hit(s) with Target = 'Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform' and Ligand = 'BDBM50427453' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50427453
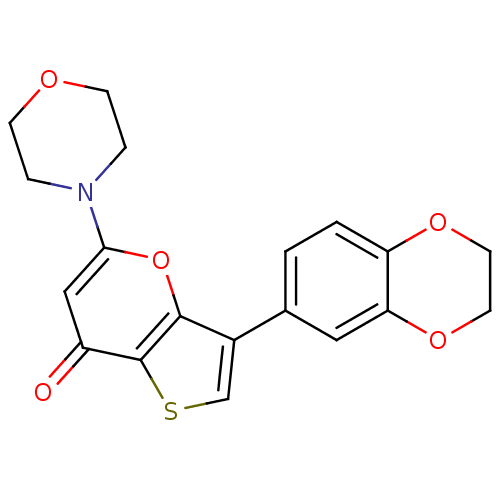
(CHEMBL2322228 | US10308662, Compound 28 | US950578...)Show InChI InChI=1S/C19H17NO5S/c21-14-10-17(20-3-5-22-6-4-20)25-18-13(11-26-19(14)18)12-1-2-15-16(9-12)24-8-7-23-15/h1-2,9-11H,3-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| US Patent
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
SignalRx Pharmaceuticals, Inc.
US Patent
| Assay Description
Several TP Scaffold compounds were tested for inhibition activity against isoforms of PI3K (alpha, beta, gamma, and delta isoforms) and the bromodoma... |
US Patent US9505780 (2016)
BindingDB Entry DOI: 10.7270/Q22B8WZ1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50427453

(CHEMBL2322228 | US10308662, Compound 28 | US950578...)Show InChI InChI=1S/C19H17NO5S/c21-14-10-17(20-3-5-22-6-4-20)25-18-13(11-26-19(14)18)12-1-2-15-16(9-12)24-8-7-23-15/h1-2,9-11H,3-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50427453

(CHEMBL2322228 | US10308662, Compound 28 | US950578...)Show InChI InChI=1S/C19H17NO5S/c21-14-10-17(20-3-5-22-6-4-20)25-18-13(11-26-19(14)18)12-1-2-15-16(9-12)24-8-7-23-15/h1-2,9-11H,3-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| US Patent
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Inhibitory activity can be determined routinely using known methods and also from commercial vendors offering this service for kinases and bromodomai... |
J Med Chem 50: 2647-54 (2007)
BindingDB Entry DOI: 10.7270/Q24170D7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50427453

(CHEMBL2322228 | US10308662, Compound 28 | US950578...)Show InChI InChI=1S/C19H17NO5S/c21-14-10-17(20-3-5-22-6-4-20)25-18-13(11-26-19(14)18)12-1-2-15-16(9-12)24-8-7-23-15/h1-2,9-11H,3-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110gamma (unknown origin) |
J Med Chem 56: 1922-39 (2013)
Article DOI: 10.1021/jm301522m
BindingDB Entry DOI: 10.7270/Q2Z89DR9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data