

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50008999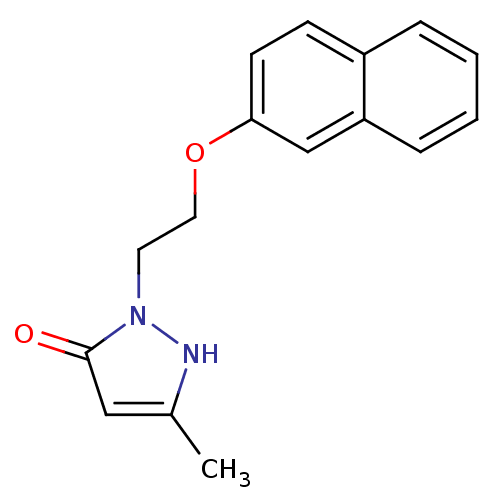 (5-Methyl-2-[2-(naphthalen-2-yloxy)-ethyl]-2,4-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [14C]-arachidonic acid conversion to 5-HETE by broken cell 5-LO isolated from guinea pig PMN | Bioorg Med Chem Lett 2: 59-62 (1992) Article DOI: 10.1016/S0960-894X(00)80655-1 BindingDB Entry DOI: 10.7270/Q2FB52TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50008999 (5-Methyl-2-[2-(naphthalen-2-yloxy)-ethyl]-2,4-dihy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches de Vitry Curated by ChEMBL | Assay Description Iin vitro inhibition of 5-lipoxygenase activity in rat basophil leukemia type 1(RBL1) cell homogenates, (reduction of [14C]-5-HETE formation) | J Med Chem 33: 2744-9 (1990) BindingDB Entry DOI: 10.7270/Q2MK6BVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50008999 (5-Methyl-2-[2-(naphthalen-2-yloxy)-ethyl]-2,4-dihy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in rat RBL-1 cells | J Med Chem 33: 1163-70 (1990) BindingDB Entry DOI: 10.7270/Q2S75F9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50008999 (5-Methyl-2-[2-(naphthalen-2-yloxy)-ethyl]-2,4-dihy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches de Vitry Curated by ChEMBL | Assay Description In vitro inhibition of 5-lipoxygenase in rat (peritoneal assay) | J Med Chem 33: 2744-9 (1990) BindingDB Entry DOI: 10.7270/Q2MK6BVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50008999 (5-Methyl-2-[2-(naphthalen-2-yloxy)-ethyl]-2,4-dihy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharma Curated by ChEMBL | Assay Description In vitro inhibition of LTB4 production was measured in rat blood | J Med Chem 34: 1028-36 (1991) BindingDB Entry DOI: 10.7270/Q28G8MXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50008999 (5-Methyl-2-[2-(naphthalen-2-yloxy)-ethyl]-2,4-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | >3.72E+5 | n/a | n/a | n/a | n/a |
ICI Pharma Curated by ChEMBL | Assay Description Ex vivo inhibition of LTB4 production was measured in dog blood | J Med Chem 34: 1028-36 (1991) BindingDB Entry DOI: 10.7270/Q28G8MXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||