

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase ROS (Homo sapiens (Human)) | BDBM267438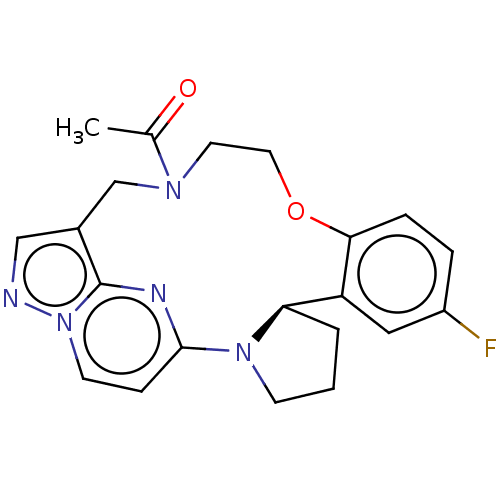 (US10688100, Compound 22 | US10966985, Compound 22 ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 331 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc. US Patent | Assay Description The potency of a compound inhibiting wild type and exemplary mutant ROS1 kinases was determined using CisBio's HTRF Kinease-TK assay technology. ... | US Patent US10966985 (2021) BindingDB Entry DOI: 10.7270/Q2639ST6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase ROS (Homo sapiens (Human)) | BDBM267438 (US10688100, Compound 22 | US10966985, Compound 22 ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 331 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc. US Patent | Assay Description The potency of a compound inhibiting wild type and exemplary mutant ROS1 kinases was determined using CisBio's HTRF Kinease-TK assay technology. ... | US Patent US10688100 (2020) BindingDB Entry DOI: 10.7270/Q2KH0RC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||