

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50437436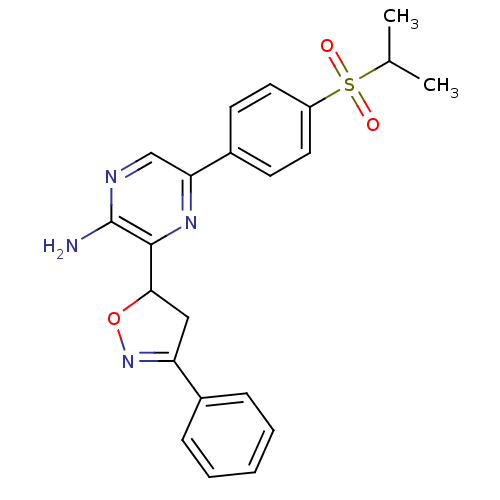 (CHEMBL2409216 | US8841450, I-1) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 16 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays were carried out in a mixtu... | US Patent US8841450 (2014) BindingDB Entry DOI: 10.7270/Q2NS0SKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50437436 (CHEMBL2409216 | US8841450, I-1) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant human full-length N-terminal Flag epitope-tagged ATR expressed in HEK293T cells using ASELPASQPQPFSAKKK peptide as substrat... | J Med Chem 62: 5547-5561 (2019) Article DOI: 10.1021/acs.jmedchem.9b00426 BindingDB Entry DOI: 10.7270/Q2BZ69C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50437436 (CHEMBL2409216 | US8841450, I-1) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Therachem Research Medilab (India) Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of ATR (unknown origin) | ACS Med Chem Lett 4: 688-9 (2013) Article DOI: 10.1021/ml4002198 BindingDB Entry DOI: 10.7270/Q2W097CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50437436 (CHEMBL2409216 | US8841450, I-1) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of ATR in human HCT116 cells assessed as reduction in histone H2AX phosphorylation by Hoechst staining-based immunofluorescence microscopi... | J Med Chem 62: 5547-5561 (2019) Article DOI: 10.1021/acs.jmedchem.9b00426 BindingDB Entry DOI: 10.7270/Q2BZ69C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||