

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18918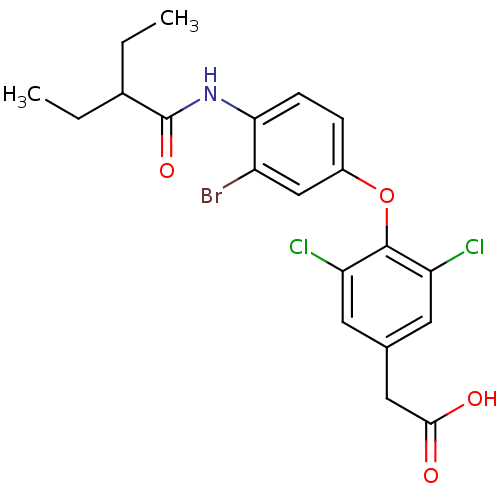 (2-{4-[3-bromo-4-(2-ethylbutanamido)phenoxy]-3,5-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Insubria Curated by ChEMBL | Assay Description Inhibition of human thyroid hormone receptor beta 1 | Bioorg Med Chem 15: 5251-61 (2007) Article DOI: 10.1016/j.bmc.2007.05.016 BindingDB Entry DOI: 10.7270/Q2PK0HD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18918 (2-{4-[3-bromo-4-(2-ethylbutanamido)phenoxy]-3,5-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo Curated by ChEMBL | Assay Description Inhibition of thyroid hormone receptor beta | Bioorg Med Chem 15: 4609-17 (2007) Article DOI: 10.1016/j.bmc.2007.04.015 BindingDB Entry DOI: 10.7270/Q2W37XJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18918 (2-{4-[3-bromo-4-(2-ethylbutanamido)phenoxy]-3,5-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB | Assay Description IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. | Bioorg Med Chem Lett 16: 884-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.002 BindingDB Entry DOI: 10.7270/Q2BV7DWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||