

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50442994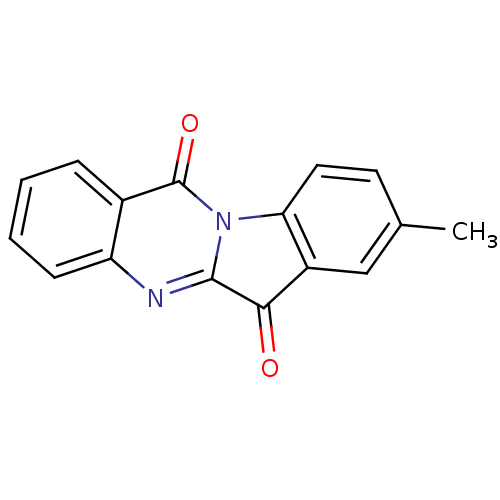 (CHEMBL312537 | US10669273, Compound 5b) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 834 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Mixed type uncompetitive inhibition of recombinant full length C-terminal His-tagged human TDO expressed in Escherichia coli using L-Trp as substrate... | Eur J Med Chem 160: 133-145 (2018) Article DOI: 10.1016/j.ejmech.2018.10.017 BindingDB Entry DOI: 10.7270/Q2GB27GB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50442994 (CHEMBL312537 | US10669273, Compound 5b) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 184 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of TDO in human U87 MG cells using L-Trp as substrate after 8 hrs | Eur J Med Chem 160: 133-145 (2018) Article DOI: 10.1016/j.ejmech.2018.10.017 BindingDB Entry DOI: 10.7270/Q2GB27GB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50442994 (CHEMBL312537 | US10669273, Compound 5b) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 215 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of TDO (unknown origin) expressed in HEK293 cells using L-Trp as substrate after 8 hrs | Eur J Med Chem 160: 133-145 (2018) Article DOI: 10.1016/j.ejmech.2018.10.017 BindingDB Entry DOI: 10.7270/Q2GB27GB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50442994 (CHEMBL312537 | US10669273, Compound 5b) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant full length C-terminal His-tagged human TDO expressed in Escherichia coli using L-Trp as substrate after 30 mins | Eur J Med Chem 160: 133-145 (2018) Article DOI: 10.1016/j.ejmech.2018.10.017 BindingDB Entry DOI: 10.7270/Q2GB27GB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||