

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50040439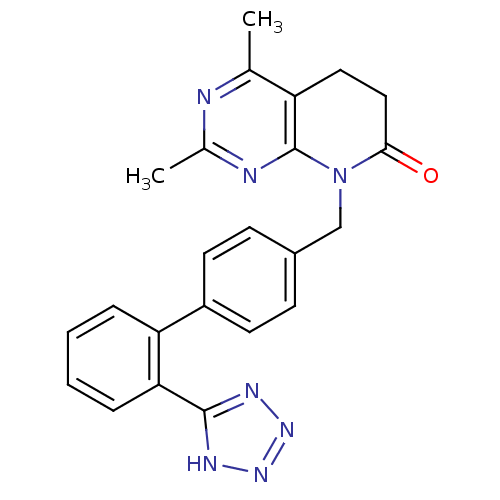 (2,4-Dimethyl-8-[2'-(1H-tetrazol-5-yl)-biphenyl-4-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Compound was evaluated for the in vitro inhibition of specific binding of [125I]-A II to Angiotensin II receptor, type 1 from rat adrenal membranes. | J Med Chem 41: 4251-60 (1998) Article DOI: 10.1021/jm970690q BindingDB Entry DOI: 10.7270/Q2FB55NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50040439 (2,4-Dimethyl-8-[2'-(1H-tetrazol-5-yl)-biphenyl-4-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of specific binding of [125 I] A II to rat liver membrane. | J Med Chem 37: 542-50 (1994) Article DOI: 10.1021/jm00030a013 BindingDB Entry DOI: 10.7270/Q2R78HX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||