

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165253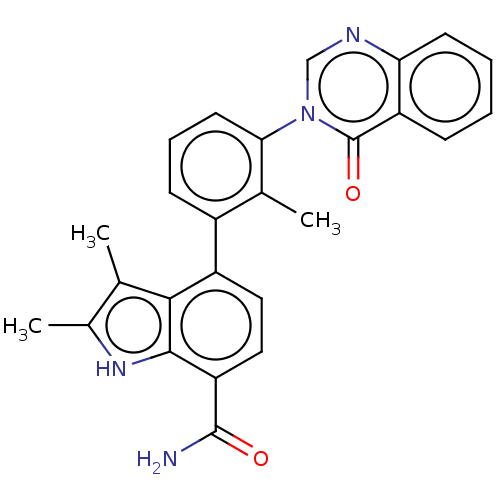 (US10604504, Example 49 | US11623921, Example 49 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description To V-bottom 384-well plates were added test compounds, human recombinant Btk (1 nM, Invitrogen Corporation), fluoresceinated peptide (1.5 μM), A... | US Patent US9688629 (2017) BindingDB Entry DOI: 10.7270/Q20863G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165253 (US10604504, Example 49 | US11623921, Example 49 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To V-bottom 384-well plates were added test compounds, human recombinant Btk (1 nM, Invitrogen Corporation), fluoresceinated peptide (1.5 μM), A... | US Patent US9802915 (2017) BindingDB Entry DOI: 10.7270/Q2JW8H17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165253 (US10604504, Example 49 | US11623921, Example 49 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description To V-bottom 384-well plates were added test compounds, human recombinant Btk (1 nM, Invitrogen Corporation), fluoresceinated peptide (1.5 μM), A... | J Med Chem 50: 4928-38 (2007) BindingDB Entry DOI: 10.7270/Q2474D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165253 (US10604504, Example 49 | US11623921, Example 49 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2X06C0T | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165253 (US10604504, Example 49 | US11623921, Example 49 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of recombinant human full-length His-tagged BTK cytoplasmic domain expressed in baculovirus expression system using fluorescence-labelled ... | Bioorg Med Chem Lett 28: 3080-3084 (2018) Article DOI: 10.1016/j.bmcl.2018.07.041 BindingDB Entry DOI: 10.7270/Q2NZ8B9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165253 (US10604504, Example 49 | US11623921, Example 49 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To V-bottom 384-well plates were added test compounds, human recombinant Btk (1 nM, Invitrogen Corporation), fluoresceinated peptide (1.5 μM), A... | US Patent US10604504 (2020) BindingDB Entry DOI: 10.7270/Q2H70JT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165253 (US10604504, Example 49 | US11623921, Example 49 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of BTK in human Ramos cells assessed as reduction in intracellular calcium level incubated for 1 hr measured for 180 secs by FLIPR assay | Bioorg Med Chem Lett 28: 3080-3084 (2018) Article DOI: 10.1016/j.bmcl.2018.07.041 BindingDB Entry DOI: 10.7270/Q2NZ8B9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||