

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM497131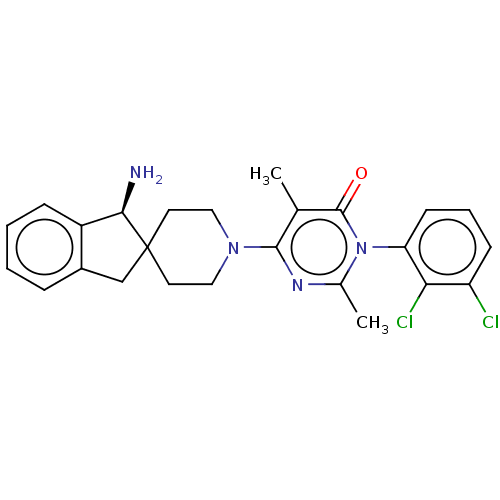 (US11001561, Compound 106) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at recombinant human N-terminal His6-tagged full length SHP2 expressed in Escherichia coli using DiFMUP as substrate measured aft... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00621 BindingDB Entry DOI: 10.7270/Q2ST7TFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM497131 (US11001561, Compound 106) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at SHP2 in human KYSE520 cells assessed as inhibition of ERK1/2 phosphorylation incubated for 2 hrs | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00621 BindingDB Entry DOI: 10.7270/Q2ST7TFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM497131 (US11001561, Compound 106) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH US Patent | Assay Description The inhibition of SHP2 by compounds of the invention was monitored using the surrogate substrate DiFMUP after protein activation by a peptide bearing... | US Patent US11001561 (2021) BindingDB Entry DOI: 10.7270/Q2BC42PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||