

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urotensin-2 receptor (RAT) | BDBM50269951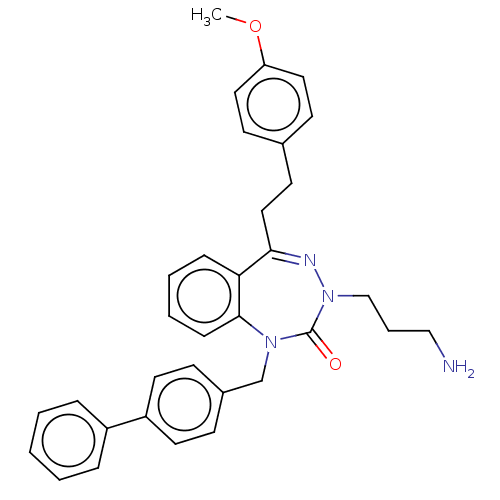 (CHEMBL4069649) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in URP-mediated aortic ring contraction by meas... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269951 (CHEMBL4069649) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in human urotensin-2-mediated aortic ring contr... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||