

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM50456701 (CHEMBL4218651) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BuChE assessed as enzyme-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Burk plot an... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456715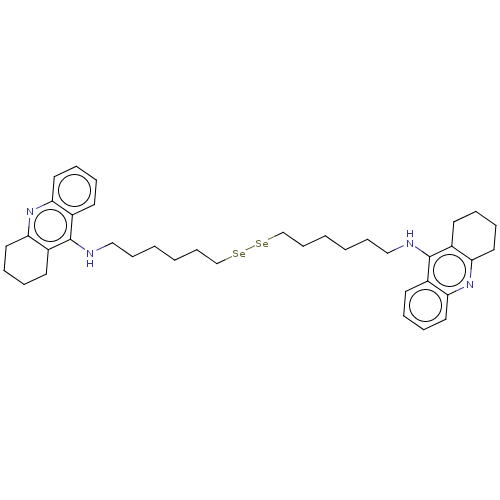 (CHEMBL4213577) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BuChE assessed as enzyme-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Burk plot an... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456702 (CHEMBL4213042) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BuChE assessed as enzyme-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Burk plot an... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456711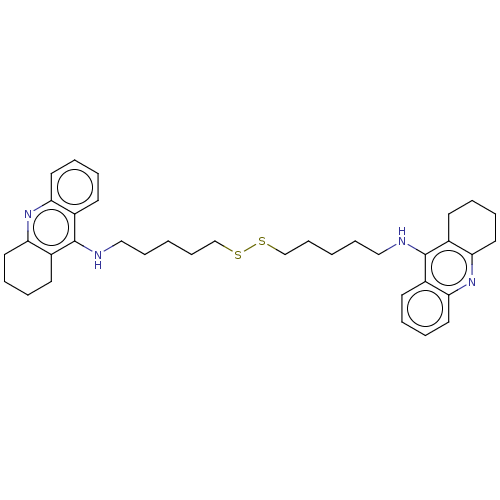 (CHEMBL4208641) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 166 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BuChE assessed as enzyme-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Burk plot an... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456712 (CHEMBL4204015) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 231 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BuChE assessed as enzyme-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Burk plot an... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456714 (CHEMBL4217969) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 243 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BuChE assessed as enzyme-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Burk plot an... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456703 (CHEMBL4209181) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 252 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BuChE assessed as enzyme-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Burk plot an... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456713 (CHEMBL4214235) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 336 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BuChE assessed as enzyme-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Burk plot an... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456708 (CHEMBL4204315) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BuChE assessed as enzyme-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Burk plot an... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456705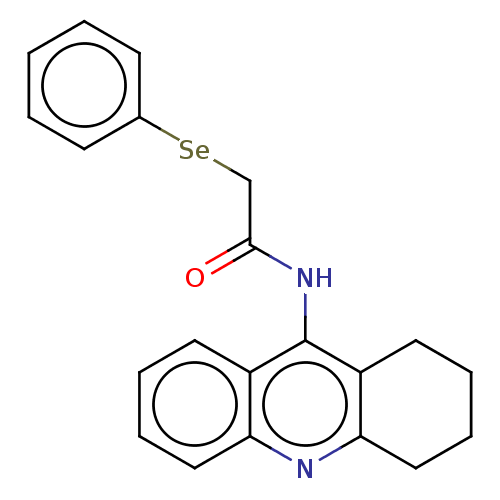 (CHEMBL4209832) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 518 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BuChE assessed as enzyme-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Burk plot an... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||