 Found 18 hits Enz. Inhib. hit(s) with all data for assayid = 1 entry = 50009489
Found 18 hits Enz. Inhib. hit(s) with all data for assayid = 1 entry = 50009489 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50073179
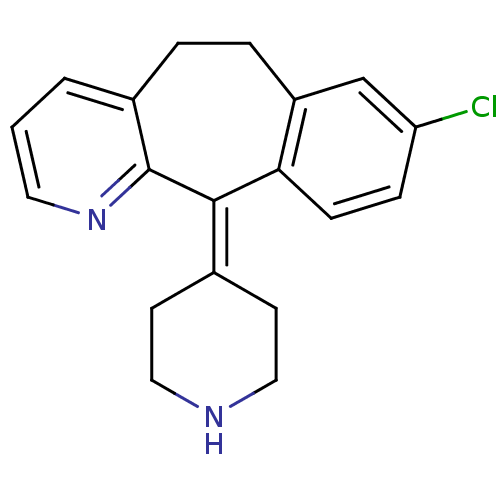
(8-Chloro-11-piperidin-4-ylidene-6,11-dihydro-5H-be...)Show SMILES Clc1ccc2c(-[#6]-[#6]-c3cccnc3\[#6]-2=[#6]-2/[#6]-[#6]-[#7]-[#6]-[#6]-2)c1 Show InChI InChI=1S/C19H19ClN2/c20-16-5-6-17-15(12-16)4-3-14-2-1-9-22-19(14)18(17)13-7-10-21-11-8-13/h1-2,5-6,9,12,21H,3-4,7-8,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126712
BindingDB Entry DOI: 10.7270/Q2736VDT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50529278
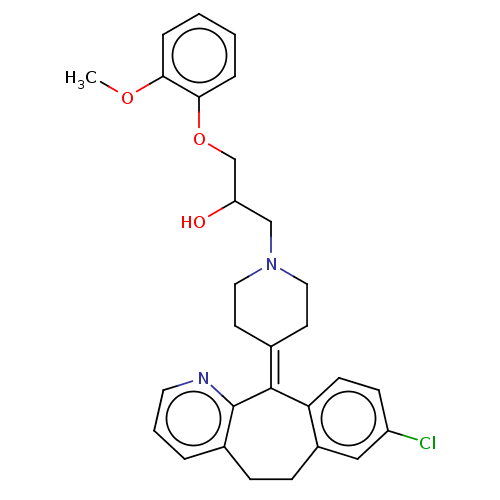
(CHEMBL4446961)Show SMILES [#6]-[#8]-c1ccccc1-[#8]-[#6]-[#6](-[#8])-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2cccnc-12 Show InChI InChI=1S/C29H31ClN2O3/c1-34-26-6-2-3-7-27(26)35-19-24(33)18-32-15-12-20(13-16-32)28-25-11-10-23(30)17-22(25)9-8-21-5-4-14-31-29(21)28/h2-7,10-11,14,17,24,33H,8-9,12-13,15-16,18-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126712
BindingDB Entry DOI: 10.7270/Q2736VDT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50529267

(CHEMBL4591702)Show SMILES [#6]-[#8]-c1ccccc1-[#8]-[#6]-[#6@H](-[#8])-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2cccnc-12 |r| Show InChI InChI=1S/C29H31ClN2O3/c1-34-26-6-2-3-7-27(26)35-19-24(33)18-32-15-12-20(13-16-32)28-25-11-10-23(30)17-22(25)9-8-21-5-4-14-31-29(21)28/h2-7,10-11,14,17,24,33H,8-9,12-13,15-16,18-19H2,1H3/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126712
BindingDB Entry DOI: 10.7270/Q2736VDT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50529266
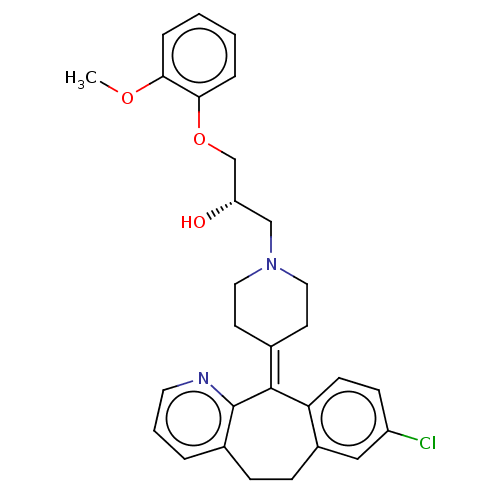
(CHEMBL4467816)Show SMILES [#6]-[#8]-c1ccccc1-[#8]-[#6]-[#6@@H](-[#8])-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2cccnc-12 |r| Show InChI InChI=1S/C29H31ClN2O3/c1-34-26-6-2-3-7-27(26)35-19-24(33)18-32-15-12-20(13-16-32)28-25-11-10-23(30)17-22(25)9-8-21-5-4-14-31-29(21)28/h2-7,10-11,14,17,24,33H,8-9,12-13,15-16,18-19H2,1H3/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126712
BindingDB Entry DOI: 10.7270/Q2736VDT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50529273

(CHEMBL4556553)Show SMILES [#8]-[#6](-[#6]-[#8]-c1ccccc1F)-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2cccnc-12 Show InChI InChI=1S/C28H28ClFN2O2/c29-22-9-10-24-21(16-22)8-7-20-4-3-13-31-28(20)27(24)19-11-14-32(15-12-19)17-23(33)18-34-26-6-2-1-5-25(26)30/h1-6,9-10,13,16,23,33H,7-8,11-12,14-15,17-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126712
BindingDB Entry DOI: 10.7270/Q2736VDT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50529276

(CHEMBL4587694)Show SMILES [#8]-[#6](-[#6]-[#8]-c1ccccc1)-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2cccnc-12 Show InChI InChI=1S/C28H29ClN2O2/c29-23-10-11-26-22(17-23)9-8-21-5-4-14-30-28(21)27(26)20-12-15-31(16-13-20)18-24(32)19-33-25-6-2-1-3-7-25/h1-7,10-11,14,17,24,32H,8-9,12-13,15-16,18-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126712
BindingDB Entry DOI: 10.7270/Q2736VDT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50529269
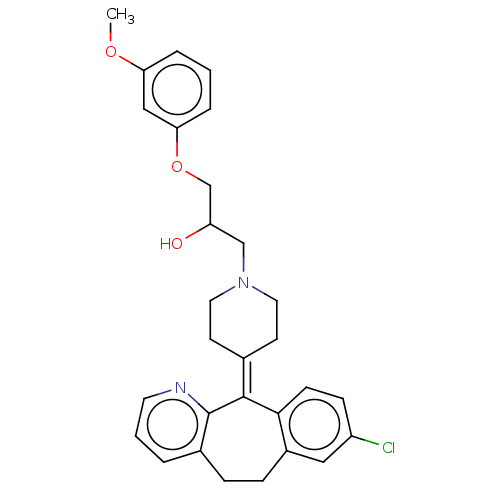
(CHEMBL4451332)Show SMILES [#6]-[#8]-c1cccc(-[#8]-[#6]-[#6](-[#8])-[#6]-[#7]-2-[#6]-[#6]\[#6](-[#6]-[#6]-2)=[#6]-2\c3ccc(Cl)cc3-[#6]-[#6]-c3cccnc-23)c1 Show InChI InChI=1S/C29H31ClN2O3/c1-34-25-5-2-6-26(17-25)35-19-24(33)18-32-14-11-20(12-15-32)28-27-10-9-23(30)16-22(27)8-7-21-4-3-13-31-29(21)28/h2-6,9-10,13,16-17,24,33H,7-8,11-12,14-15,18-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126712
BindingDB Entry DOI: 10.7270/Q2736VDT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50529275
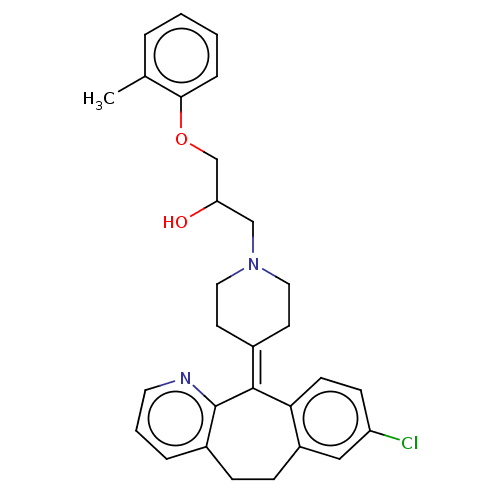
(CHEMBL4539150)Show SMILES [#6]-c1ccccc1-[#8]-[#6]-[#6](-[#8])-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2cccnc-12 Show InChI InChI=1S/C29H31ClN2O2/c1-20-5-2-3-7-27(20)34-19-25(33)18-32-15-12-21(13-16-32)28-26-11-10-24(30)17-23(26)9-8-22-6-4-14-31-29(22)28/h2-7,10-11,14,17,25,33H,8-9,12-13,15-16,18-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126712
BindingDB Entry DOI: 10.7270/Q2736VDT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50529279
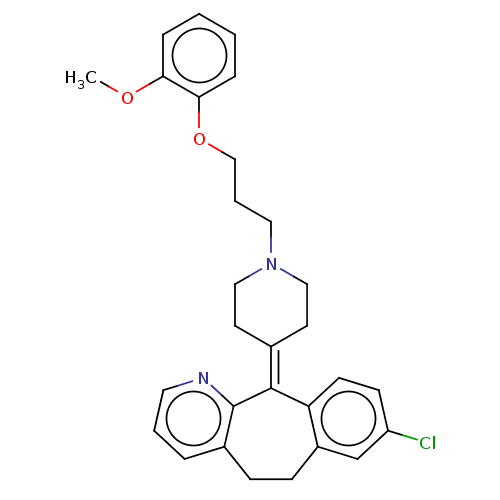
(CHEMBL4473466)Show SMILES [#6]-[#8]-c1ccccc1-[#8]-[#6]-[#6]-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2cccnc-12 Show InChI InChI=1S/C29H31ClN2O2/c1-33-26-7-2-3-8-27(26)34-19-5-16-32-17-13-21(14-18-32)28-25-12-11-24(30)20-23(25)10-9-22-6-4-15-31-29(22)28/h2-4,6-8,11-12,15,20H,5,9-10,13-14,16-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126712
BindingDB Entry DOI: 10.7270/Q2736VDT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50529280
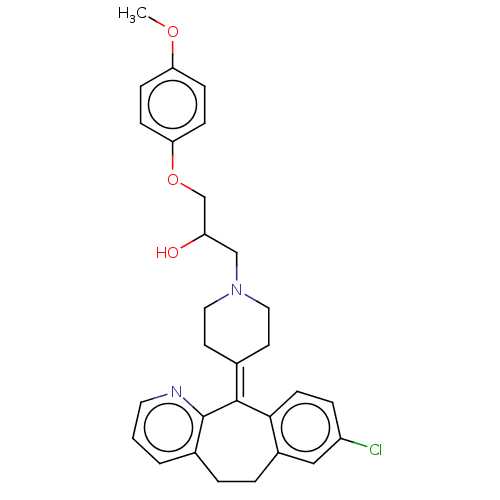
(CHEMBL4581995)Show SMILES [#6]-[#8]-c1ccc(-[#8]-[#6]-[#6](-[#8])-[#6]-[#7]-2-[#6]-[#6]\[#6](-[#6]-[#6]-2)=[#6]-2\c3ccc(Cl)cc3-[#6]-[#6]-c3cccnc-23)cc1 Show InChI InChI=1S/C29H31ClN2O3/c1-34-25-7-9-26(10-8-25)35-19-24(33)18-32-15-12-20(13-16-32)28-27-11-6-23(30)17-22(27)5-4-21-3-2-14-31-29(21)28/h2-3,6-11,14,17,24,33H,4-5,12-13,15-16,18-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126712
BindingDB Entry DOI: 10.7270/Q2736VDT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50529265
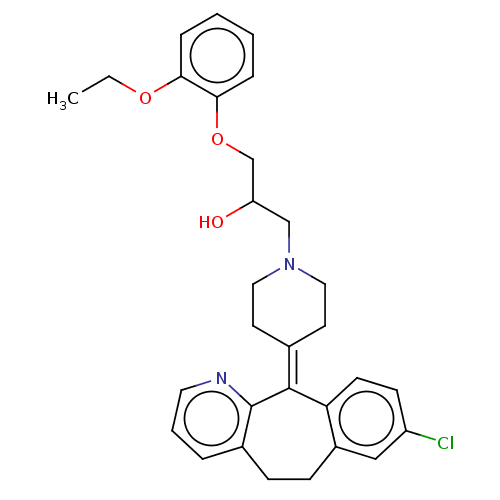
(CHEMBL4473399)Show SMILES [#6]-[#6]-[#8]-c1ccccc1-[#8]-[#6]-[#6](-[#8])-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2cccnc-12 Show InChI InChI=1S/C30H33ClN2O3/c1-2-35-27-7-3-4-8-28(27)36-20-25(34)19-33-16-13-21(14-17-33)29-26-12-11-24(31)18-23(26)10-9-22-6-5-15-32-30(22)29/h3-8,11-12,15,18,25,34H,2,9-10,13-14,16-17,19-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126712
BindingDB Entry DOI: 10.7270/Q2736VDT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50529270
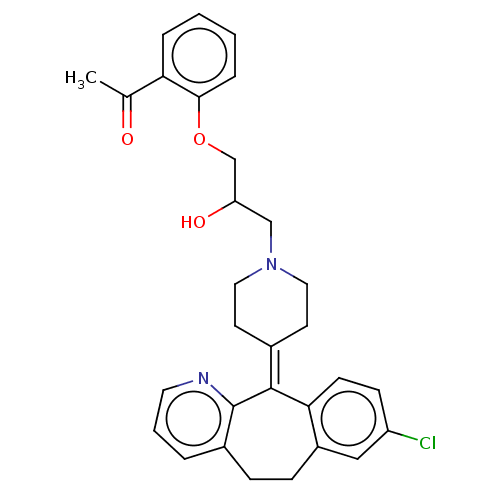
(CHEMBL4518271)Show SMILES [#6]-[#6](=O)-c1ccccc1-[#8]-[#6]-[#6](-[#8])-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2cccnc-12 Show InChI InChI=1S/C30H31ClN2O3/c1-20(34)26-6-2-3-7-28(26)36-19-25(35)18-33-15-12-21(13-16-33)29-27-11-10-24(31)17-23(27)9-8-22-5-4-14-32-30(22)29/h2-7,10-11,14,17,25,35H,8-9,12-13,15-16,18-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126712
BindingDB Entry DOI: 10.7270/Q2736VDT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50529277
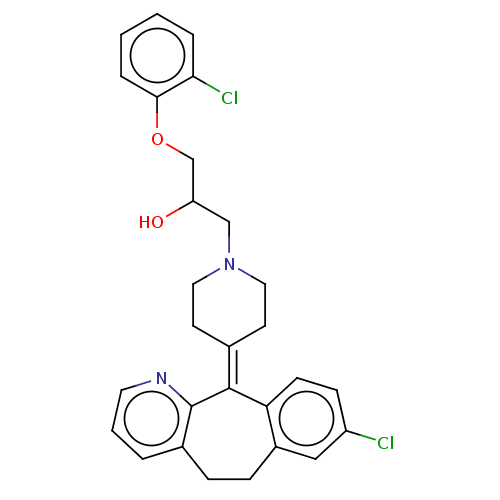
(CHEMBL4539056)Show SMILES [#8]-[#6](-[#6]-[#8]-c1ccccc1Cl)-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2cccnc-12 Show InChI InChI=1S/C28H28Cl2N2O2/c29-22-9-10-24-21(16-22)8-7-20-4-3-13-31-28(20)27(24)19-11-14-32(15-12-19)17-23(33)18-34-26-6-2-1-5-25(26)30/h1-6,9-10,13,16,23,33H,7-8,11-12,14-15,17-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126712
BindingDB Entry DOI: 10.7270/Q2736VDT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50529268
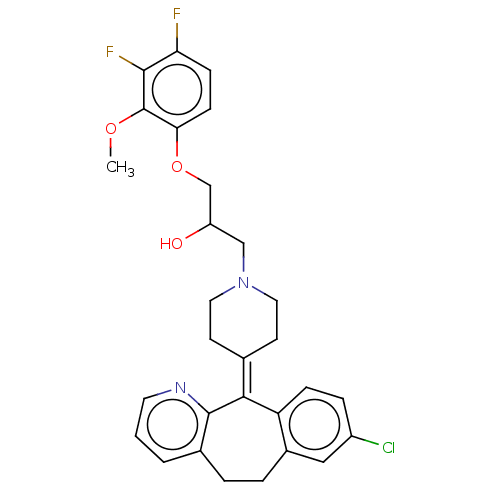
(CHEMBL4529928)Show SMILES [#6]-[#8]-c1c(F)c(F)ccc1-[#8]-[#6]-[#6](-[#8])-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2cccnc-12 Show InChI InChI=1S/C29H29ClF2N2O3/c1-36-29-25(9-8-24(31)27(29)32)37-17-22(35)16-34-13-10-18(11-14-34)26-23-7-6-21(30)15-20(23)5-4-19-3-2-12-33-28(19)26/h2-3,6-9,12,15,22,35H,4-5,10-11,13-14,16-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126712
BindingDB Entry DOI: 10.7270/Q2736VDT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50529274

(CHEMBL4557928)Show SMILES [#8]-[#6](-[#6]-[#8]-c1ccccc1-[#8]C(F)(F)F)-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2cccnc-12 Show InChI InChI=1S/C29H28ClF3N2O3/c30-22-9-10-24-21(16-22)8-7-20-4-3-13-34-28(20)27(24)19-11-14-35(15-12-19)17-23(36)18-37-25-5-1-2-6-26(25)38-29(31,32)33/h1-6,9-10,13,16,23,36H,7-8,11-12,14-15,17-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126712
BindingDB Entry DOI: 10.7270/Q2736VDT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50529272
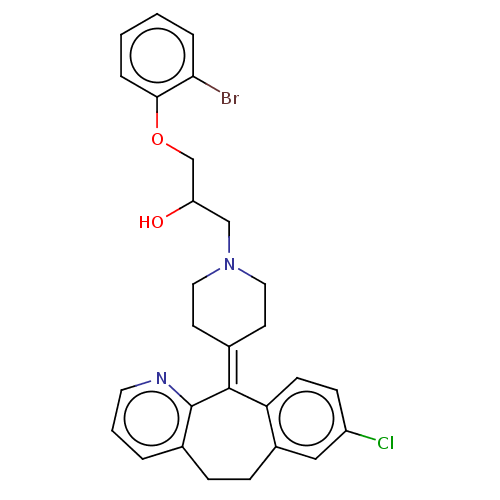
(CHEMBL4458953)Show SMILES [#8]-[#6](-[#6]-[#8]-c1ccccc1Br)-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2cccnc-12 Show InChI InChI=1S/C28H28BrClN2O2/c29-25-5-1-2-6-26(25)34-18-23(33)17-32-14-11-19(12-15-32)27-24-10-9-22(30)16-21(24)8-7-20-4-3-13-31-28(20)27/h1-6,9-10,13,16,23,33H,7-8,11-12,14-15,17-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126712
BindingDB Entry DOI: 10.7270/Q2736VDT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50529271
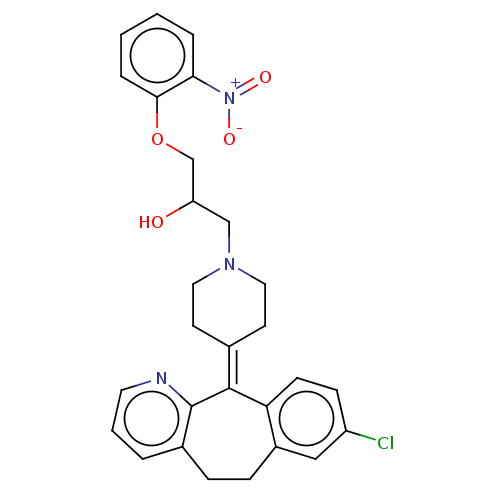
(CHEMBL4445636)Show SMILES [#8]-[#6](-[#6]-[#8]-c1ccccc1-[#7+](-[#8-])=O)-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2cccnc-12 Show InChI InChI=1S/C28H28ClN3O4/c29-22-9-10-24-21(16-22)8-7-20-4-3-13-30-28(20)27(24)19-11-14-31(15-12-19)17-23(33)18-36-26-6-2-1-5-25(26)32(34)35/h1-6,9-10,13,16,23,33H,7-8,11-12,14-15,17-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126712
BindingDB Entry DOI: 10.7270/Q2736VDT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50529281
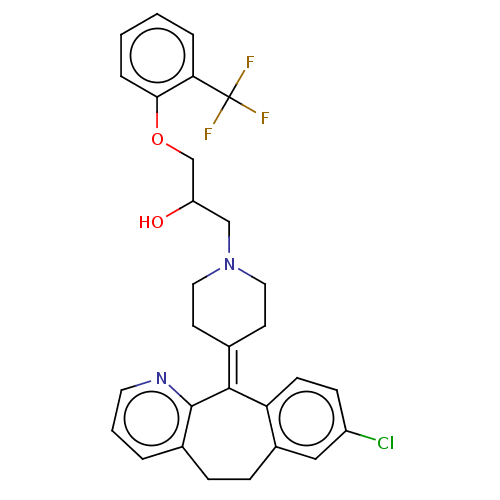
(CHEMBL4565774)Show SMILES [#8]-[#6](-[#6]-[#8]-c1ccccc1C(F)(F)F)-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2cccnc-12 Show InChI InChI=1S/C29H28ClF3N2O2/c30-22-9-10-24-21(16-22)8-7-20-4-3-13-34-28(20)27(24)19-11-14-35(15-12-19)17-23(36)18-37-26-6-2-1-5-25(26)29(31,32)33/h1-6,9-10,13,16,23,36H,7-8,11-12,14-15,17-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Antagonist activity at histamine H1 receptor (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126712
BindingDB Entry DOI: 10.7270/Q2736VDT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data