 Found 20 hits Enz. Inhib. hit(s) with all data for assayid = 1 entry = 50018882
Found 20 hits Enz. Inhib. hit(s) with all data for assayid = 1 entry = 50018882 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM323704
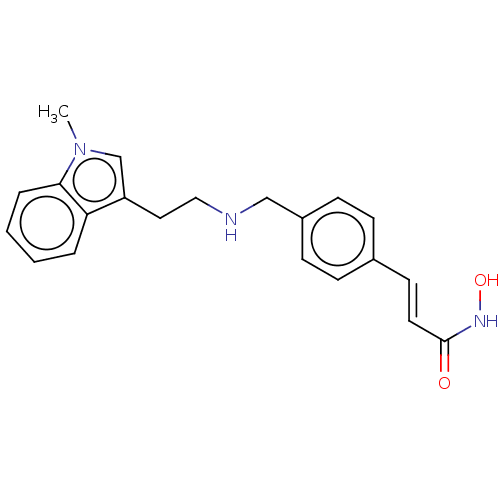
(US10188756, Compound CN107)Show InChI InChI=1S/C21H23N3O2/c1-24-15-18(19-4-2-3-5-20(19)24)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)23-26/h2-11,15,22,26H,12-14H2,1H3,(H,23,25)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM323702
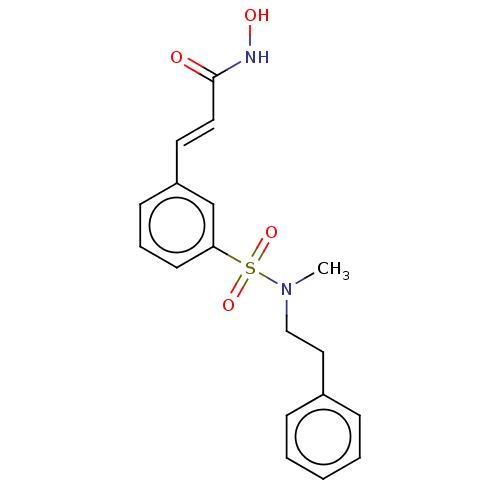
(US10188756, Compound CN89)Show SMILES CN(CCc1ccccc1)S(=O)(=O)c1cccc(\C=C\C(=O)NO)c1 Show InChI InChI=1S/C18H20N2O4S/c1-20(13-12-15-6-3-2-4-7-15)25(23,24)17-9-5-8-16(14-17)10-11-18(21)19-22/h2-11,14,22H,12-13H2,1H3,(H,19,21)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50613612

(CHEMBL5283887) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50613615

(CHEMBL5265931)Show SMILES ONC(=O)c1ccc(NC(=O)CCc2ccc(Oc3cccs3)c(O)c2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50332494
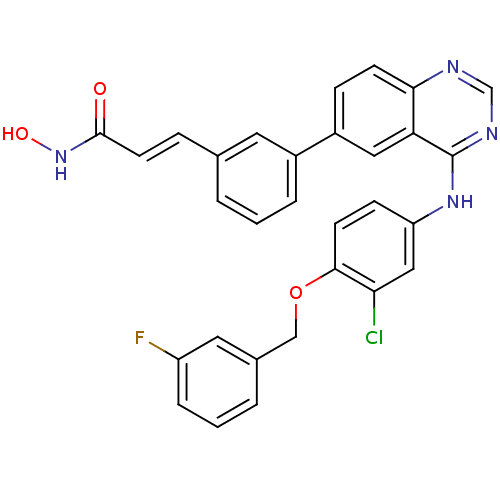
((E)-3-{3-[4-(3-Chloro-4-(3-fluorobenzyloxy)phenyla...)Show SMILES ONC(=O)\C=C\c1cccc(c1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C30H22ClFN4O3/c31-26-16-24(9-11-28(26)39-17-20-4-2-6-23(32)14-20)35-30-25-15-22(8-10-27(25)33-18-34-30)21-5-1-3-19(13-21)7-12-29(37)36-38/h1-16,18,38H,17H2,(H,36,37)(H,33,34,35)/b12-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50613610

(CHEMBL5270345)Show SMILES CC(C)Cc1c(OCCOc2cccc(\C=C\C(=O)NO)c2)ccc2CCC(=O)Oc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50613616

(CHEMBL5290012)Show SMILES COc1cc2c(Nc3cccc(\C=C\C(=O)NO)c3)ncnc2cc1OCCCN1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50531369

(CHEMBL4533192)Show SMILES ONC(=O)CCCCCCNC(=O)c1c(sc2cc(O)ccc12)-c1ccc(O)cc1 Show InChI InChI=1S/C22H24N2O5S/c25-15-8-6-14(7-9-15)21-20(17-11-10-16(26)13-18(17)30-21)22(28)23-12-4-2-1-3-5-19(27)24-29/h6-11,13,25-26,29H,1-5,12H2,(H,23,28)(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 168 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50613617

(CHEMBL5271319)Show SMILES ONC(=O)\C=C\c1cccc(Nc2ncnc3cc4OCCOc4cc23)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50015233
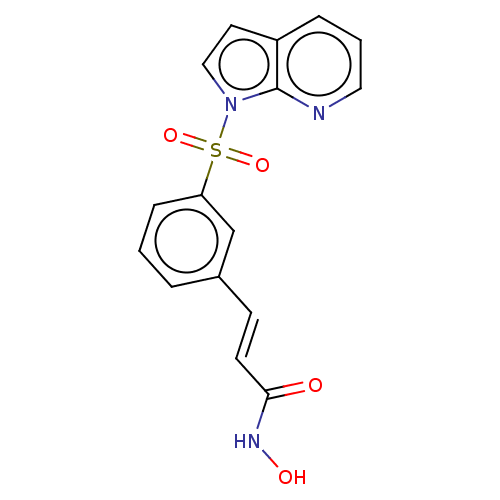
(CHEMBL3262727)Show SMILES ONC(=O)\C=C\c1cccc(c1)S(=O)(=O)n1ccc2cccnc12 Show InChI InChI=1S/C16H13N3O4S/c20-15(18-21)7-6-12-3-1-5-14(11-12)24(22,23)19-10-8-13-4-2-9-17-16(13)19/h1-11,21H,(H,18,20)/b7-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50531335

(CHEMBL4586891)Show SMILES ONC(=O)CCCCCn1cc(nn1)-c1ccc(cc1)C(=O)c1c(sc2cc(O)ccc12)-c1ccc(O)cc1 Show InChI InChI=1S/C29H26N4O5S/c34-21-11-9-20(10-12-21)29-27(23-14-13-22(35)16-25(23)39-29)28(37)19-7-5-18(6-8-19)24-17-33(32-30-24)15-3-1-2-4-26(36)31-38/h5-14,16-17,34-35,38H,1-4,15H2,(H,31,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50613613

(CHEMBL5271192) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM22449
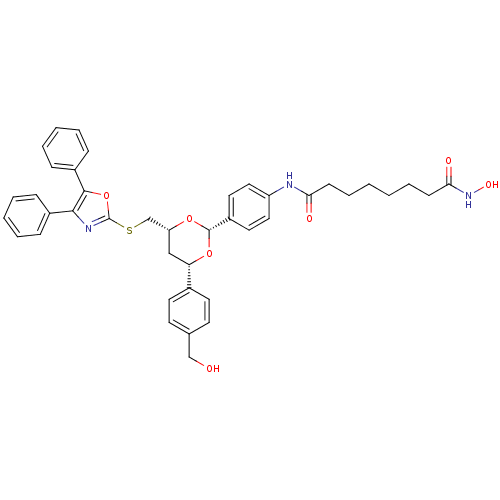
(CHEMBL356769 | N-(4-{(2R,4R,6S)-4-{[(4,5-diphenyl-...)Show SMILES OCc1ccc(cc1)[C@@H]1C[C@H](CSc2nc(c(o2)-c2ccccc2)-c2ccccc2)O[C@@H](O1)c1ccc(NC(=O)CCCCCCC(=O)NO)cc1 |r| Show InChI InChI=1S/C41H43N3O7S/c45-26-28-17-19-29(20-18-28)35-25-34(27-52-41-43-38(30-11-5-3-6-12-30)39(51-41)31-13-7-4-8-14-31)49-40(50-35)32-21-23-33(24-22-32)42-36(46)15-9-1-2-10-16-37(47)44-48/h3-8,11-14,17-24,34-35,40,45,48H,1-2,9-10,15-16,25-27H2,(H,42,46)(H,44,47)/t34-,35+,40+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50613611

(CHEMBL5270048) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM198121
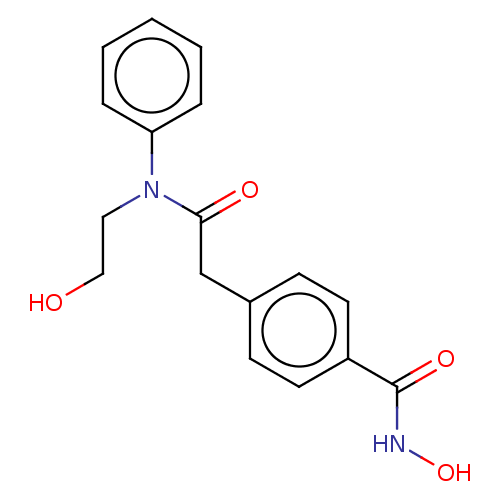
(HPOB)Show InChI InChI=1S/C17H18N2O4/c20-11-10-19(15-4-2-1-3-5-15)16(21)12-13-6-8-14(9-7-13)17(22)18-23/h1-9,20,23H,10-12H2,(H,18,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50492835
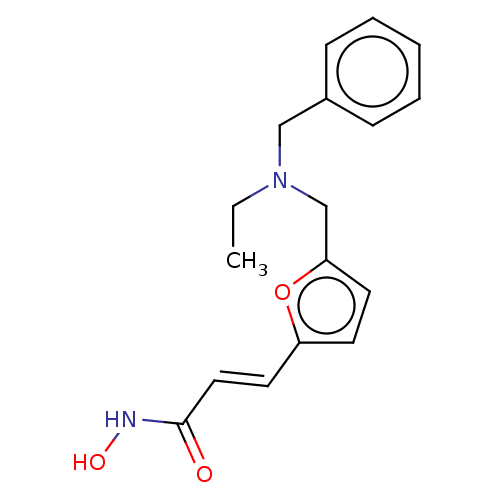
(CHEMBL2413316)Show InChI InChI=1S/C17H20N2O3/c1-2-19(12-14-6-4-3-5-7-14)13-16-9-8-15(22-16)10-11-17(20)18-21/h3-11,21H,2,12-13H2,1H3,(H,18,20)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50380399

(CHEMBL2018302 | Tubastatin A | US10227295, Compoun...)Show InChI InChI=1S/C20H21N3O2/c1-22-11-10-19-17(13-22)16-4-2-3-5-18(16)23(19)12-14-6-8-15(9-7-14)20(24)21-25/h2-9,25H,10-13H2,1H3,(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 1.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50348388
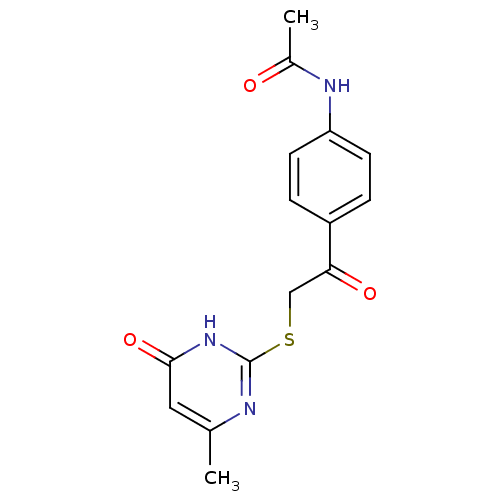
(CHEMBL1800241)Show InChI InChI=1S/C15H15N3O3S/c1-9-7-14(21)18-15(16-9)22-8-13(20)11-3-5-12(6-4-11)17-10(2)19/h3-7H,8H2,1-2H3,(H,17,19)(H,16,18,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 1.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50613614

(CHEMBL5276344) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data