

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50126143 (Epacadostat | INCB-024360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His-tagged human IDO1 expressed in Escherichia coli assessed as reduction in N-formylkynurenine formation using ... | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111629 BindingDB Entry DOI: 10.7270/Q2BZ69GN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50528763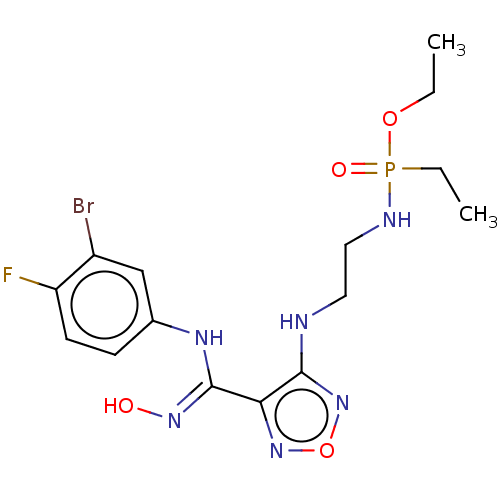 (CHEMBL4472697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His-tagged human IDO1 expressed in Escherichia coli assessed as reduction in N-formylkynurenine formation using ... | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111629 BindingDB Entry DOI: 10.7270/Q2BZ69GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50528782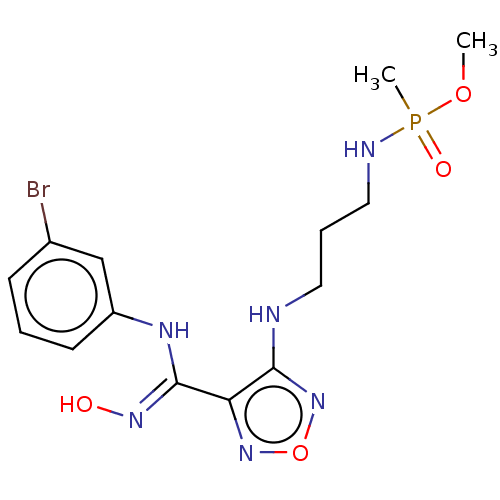 (CHEMBL4454093) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His-tagged human IDO1 expressed in Escherichia coli assessed as reduction in N-formylkynurenine formation using ... | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111629 BindingDB Entry DOI: 10.7270/Q2BZ69GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50528777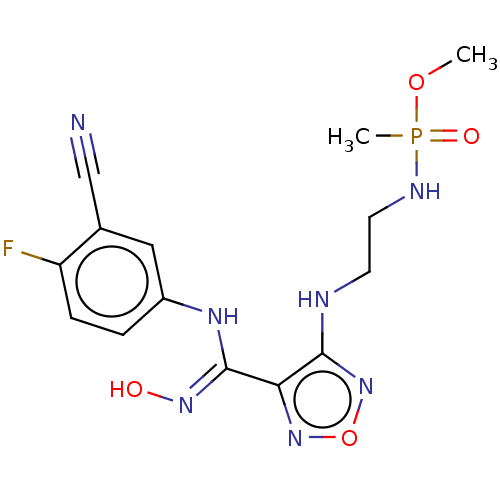 (CHEMBL4455008) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His-tagged human IDO1 expressed in Escherichia coli assessed as reduction in N-formylkynurenine formation using ... | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111629 BindingDB Entry DOI: 10.7270/Q2BZ69GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50528761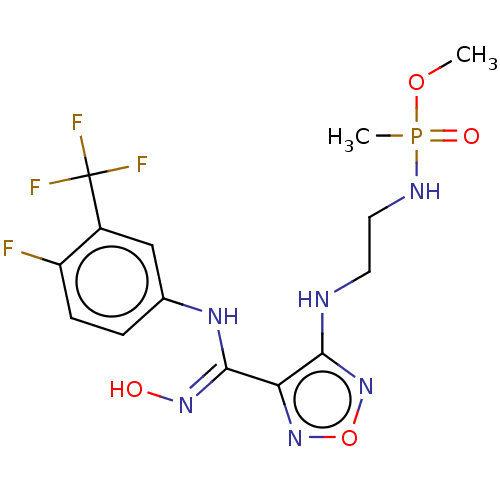 (CHEMBL4552082) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His-tagged human IDO1 expressed in Escherichia coli assessed as reduction in N-formylkynurenine formation using ... | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111629 BindingDB Entry DOI: 10.7270/Q2BZ69GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50528779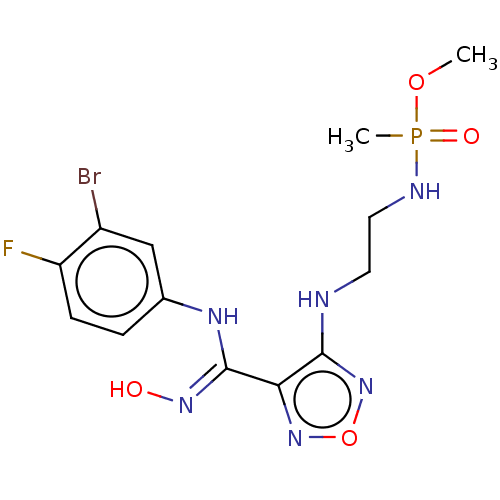 (CHEMBL4514196) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His-tagged human IDO1 expressed in Escherichia coli assessed as reduction in N-formylkynurenine formation using ... | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111629 BindingDB Entry DOI: 10.7270/Q2BZ69GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50528762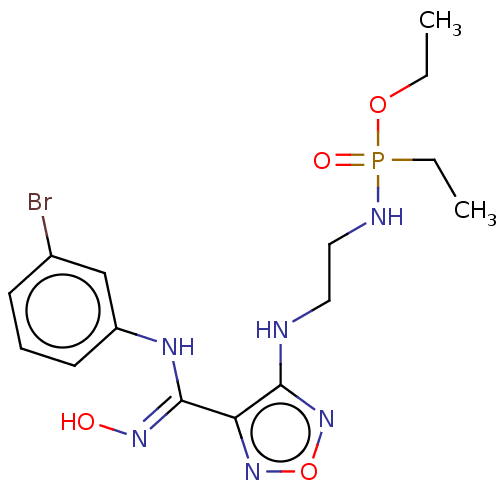 (CHEMBL4581189) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His-tagged human IDO1 expressed in Escherichia coli assessed as reduction in N-formylkynurenine formation using ... | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111629 BindingDB Entry DOI: 10.7270/Q2BZ69GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50528775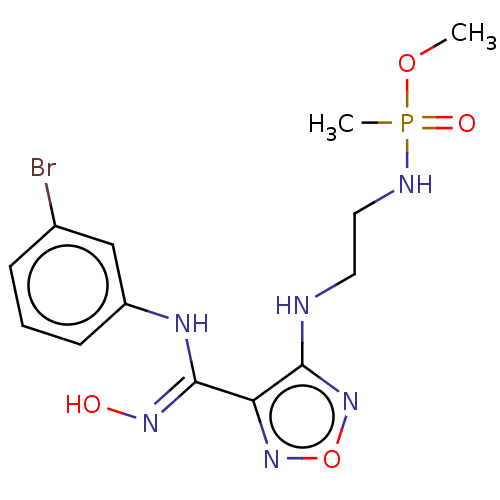 (CHEMBL4552649) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His-tagged human IDO1 expressed in Escherichia coli assessed as reduction in N-formylkynurenine formation using ... | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111629 BindingDB Entry DOI: 10.7270/Q2BZ69GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50528770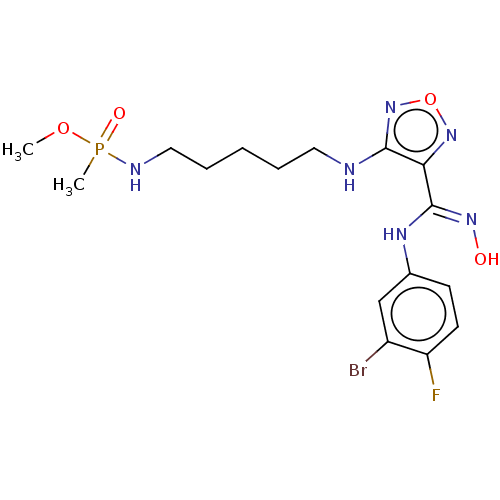 (CHEMBL4567170) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His-tagged human IDO1 expressed in Escherichia coli assessed as reduction in N-formylkynurenine formation using ... | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111629 BindingDB Entry DOI: 10.7270/Q2BZ69GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50528760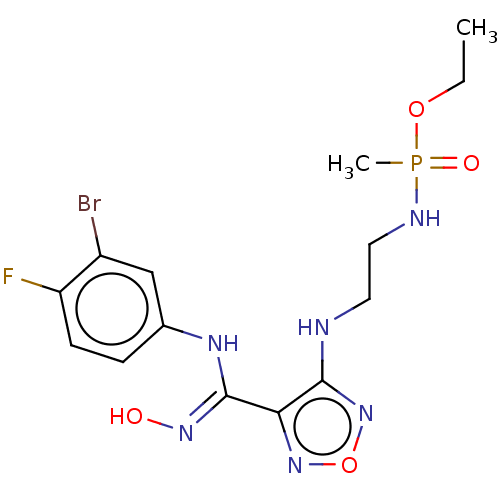 (CHEMBL4444787) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His-tagged human IDO1 expressed in Escherichia coli assessed as reduction in N-formylkynurenine formation using ... | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111629 BindingDB Entry DOI: 10.7270/Q2BZ69GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50528785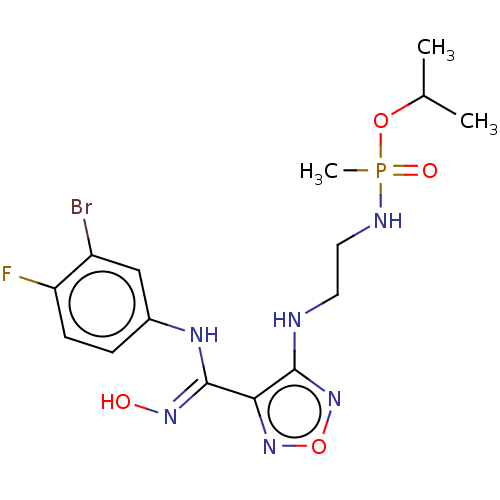 (CHEMBL4514159) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His-tagged human IDO1 expressed in Escherichia coli assessed as reduction in N-formylkynurenine formation using ... | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111629 BindingDB Entry DOI: 10.7270/Q2BZ69GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50528783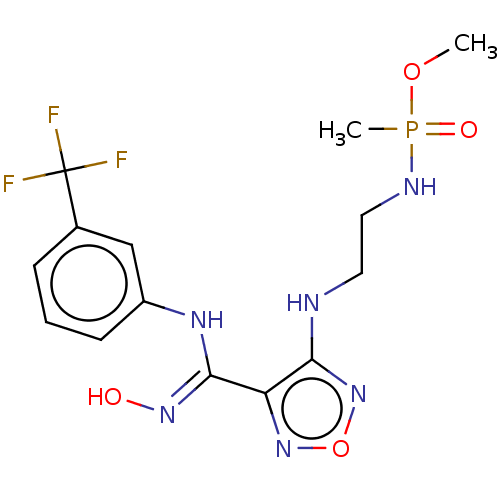 (CHEMBL4455785) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His-tagged human IDO1 expressed in Escherichia coli assessed as reduction in N-formylkynurenine formation using ... | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111629 BindingDB Entry DOI: 10.7270/Q2BZ69GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50528780 (CHEMBL4436582) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His-tagged human IDO1 expressed in Escherichia coli assessed as reduction in N-formylkynurenine formation using ... | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111629 BindingDB Entry DOI: 10.7270/Q2BZ69GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50528781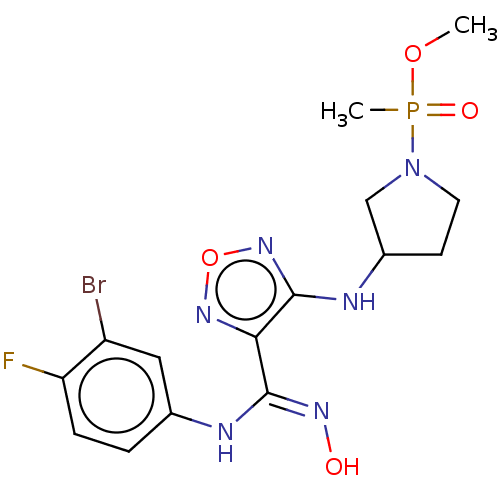 (CHEMBL4460529) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His-tagged human IDO1 expressed in Escherichia coli assessed as reduction in N-formylkynurenine formation using ... | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111629 BindingDB Entry DOI: 10.7270/Q2BZ69GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||