

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Lille-Nord de France Curated by ChEMBL | Assay Description Inhibition of COX2 in human whole blood assessed as inhibition of 12-hydroxyheptadecatrienoic acid production by HPLC method | Bioorg Med Chem 18: 3910-24 (2010) Article DOI: 10.1016/j.bmc.2010.04.034 BindingDB Entry DOI: 10.7270/Q28K7B16 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Lille-Nord de France Curated by ChEMBL | Assay Description Inhibition of COX2 in human whole blood assessed as inhibition of 12-hydroxyheptadecatrienoic acid production by HPLC method | Bioorg Med Chem 18: 3910-24 (2010) Article DOI: 10.1016/j.bmc.2010.04.034 BindingDB Entry DOI: 10.7270/Q28K7B16 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50320709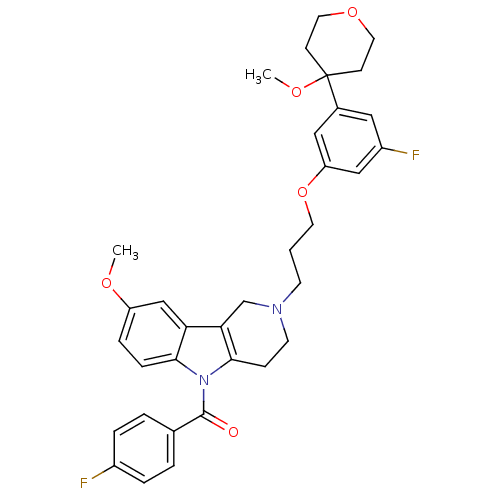 (5-(4-Fluorobenzoyl)-2-{3-[3-fluoro-5-(4-methoxytet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Lille-Nord de France Curated by ChEMBL | Assay Description Inhibition of COX2 in human whole blood assessed as inhibition of 12-hydroxyheptadecatrienoic acid production by HPLC method | Bioorg Med Chem 18: 3910-24 (2010) Article DOI: 10.1016/j.bmc.2010.04.034 BindingDB Entry DOI: 10.7270/Q28K7B16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50320705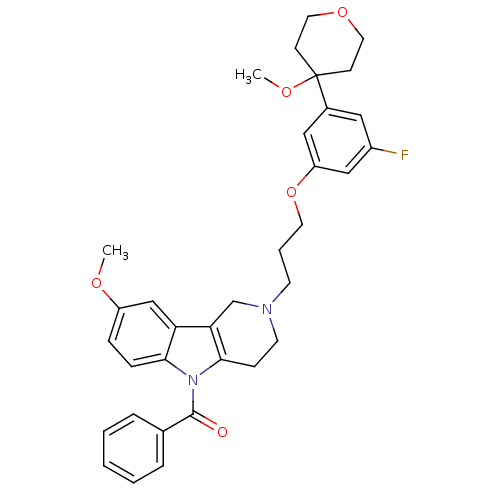 (5-Benzoyl-2-{3-[3-fluoro-5-(4-methoxytetrahydro-4H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Lille-Nord de France Curated by ChEMBL | Assay Description Inhibition of COX2 in human whole blood assessed as inhibition of 12-hydroxyheptadecatrienoic acid production by HPLC method | Bioorg Med Chem 18: 3910-24 (2010) Article DOI: 10.1016/j.bmc.2010.04.034 BindingDB Entry DOI: 10.7270/Q28K7B16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50320712 (5-(4-Bromobenzoyl)-2-{3-[3-fluoro-5-(4-methoxytetr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Lille-Nord de France Curated by ChEMBL | Assay Description Inhibition of COX2 in human whole blood assessed as inhibition of 12-hydroxyheptadecatrienoic acid production by HPLC method | Bioorg Med Chem 18: 3910-24 (2010) Article DOI: 10.1016/j.bmc.2010.04.034 BindingDB Entry DOI: 10.7270/Q28K7B16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50320703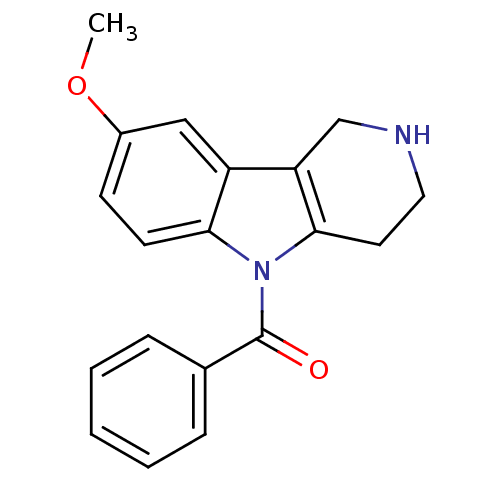 (5-Benzoyl-8-methoxy-2,3,4,5-tetrahydro-1H-pyrido[4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Lille-Nord de France Curated by ChEMBL | Assay Description Inhibition of COX2 in human whole blood assessed as inhibition of 12-hydroxyheptadecatrienoic acid production by HPLC method | Bioorg Med Chem 18: 3910-24 (2010) Article DOI: 10.1016/j.bmc.2010.04.034 BindingDB Entry DOI: 10.7270/Q28K7B16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50320711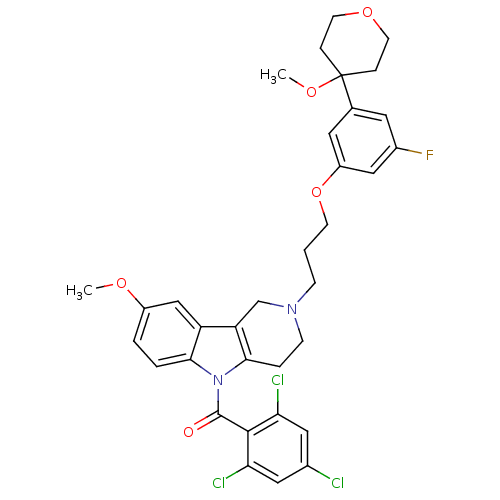 (2-{3-[3-Fluoro-5-(4-methoxytetrahydro-4H-4-pyranyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Lille-Nord de France Curated by ChEMBL | Assay Description Inhibition of COX2 in human whole blood assessed as inhibition of 12-hydroxyheptadecatrienoic acid production by HPLC method | Bioorg Med Chem 18: 3910-24 (2010) Article DOI: 10.1016/j.bmc.2010.04.034 BindingDB Entry DOI: 10.7270/Q28K7B16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50320706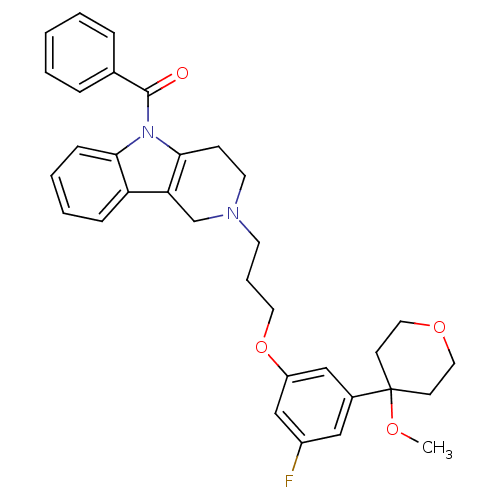 (5-Benzoyl-2-{3-[3-fluoro-5-(4-methoxytetrahydro-4H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Lille-Nord de France Curated by ChEMBL | Assay Description Inhibition of COX2 in human whole blood assessed as inhibition of 12-hydroxyheptadecatrienoic acid production by HPLC method | Bioorg Med Chem 18: 3910-24 (2010) Article DOI: 10.1016/j.bmc.2010.04.034 BindingDB Entry DOI: 10.7270/Q28K7B16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50320714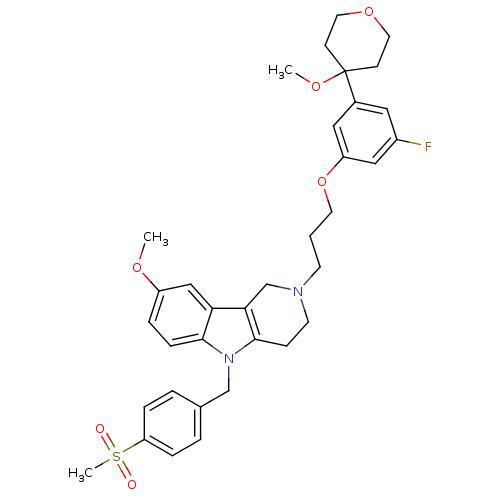 (2-{3-[3-Fluoro-5-(4-methoxytetrahydro-4H-4-pyranyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.09E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Lille-Nord de France Curated by ChEMBL | Assay Description Inhibition of COX2 in human whole blood assessed as inhibition of 12-hydroxyheptadecatrienoic acid production by HPLC method | Bioorg Med Chem 18: 3910-24 (2010) Article DOI: 10.1016/j.bmc.2010.04.034 BindingDB Entry DOI: 10.7270/Q28K7B16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50320707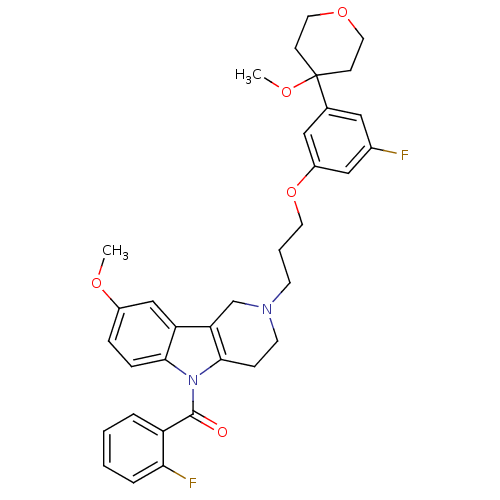 (5-(2-Fluorobenzoyl)-2-{3-[3-fluoro-5-(4-methoxytet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.26E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Lille-Nord de France Curated by ChEMBL | Assay Description Inhibition of COX2 in human whole blood assessed as inhibition of 12-hydroxyheptadecatrienoic acid production by HPLC method | Bioorg Med Chem 18: 3910-24 (2010) Article DOI: 10.1016/j.bmc.2010.04.034 BindingDB Entry DOI: 10.7270/Q28K7B16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50320710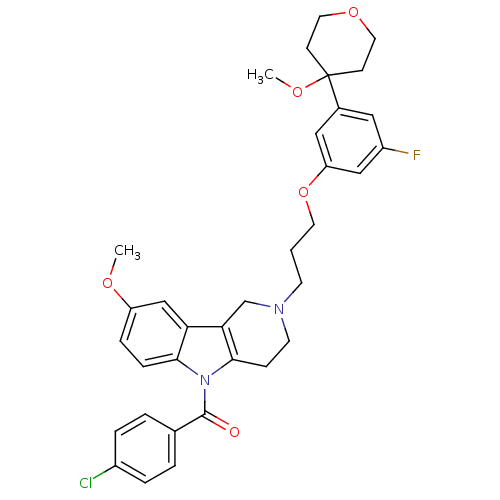 (5-(4-Chlorobenzoyl)-2-{3-[3-fluoro-5-(4-methoxytet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.98E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Lille-Nord de France Curated by ChEMBL | Assay Description Inhibition of COX2 in human whole blood assessed as inhibition of 12-hydroxyheptadecatrienoic acid production by HPLC method | Bioorg Med Chem 18: 3910-24 (2010) Article DOI: 10.1016/j.bmc.2010.04.034 BindingDB Entry DOI: 10.7270/Q28K7B16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50320708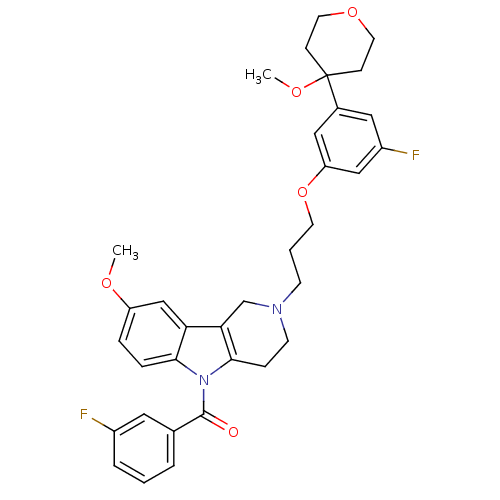 (5-(3-Fluorobenzoyl)-2-{3-[3-fluoro-5-(4-methoxytet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.57E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Lille-Nord de France Curated by ChEMBL | Assay Description Inhibition of COX2 in human whole blood assessed as inhibition of 12-hydroxyheptadecatrienoic acid production by HPLC method | Bioorg Med Chem 18: 3910-24 (2010) Article DOI: 10.1016/j.bmc.2010.04.034 BindingDB Entry DOI: 10.7270/Q28K7B16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||