

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM255476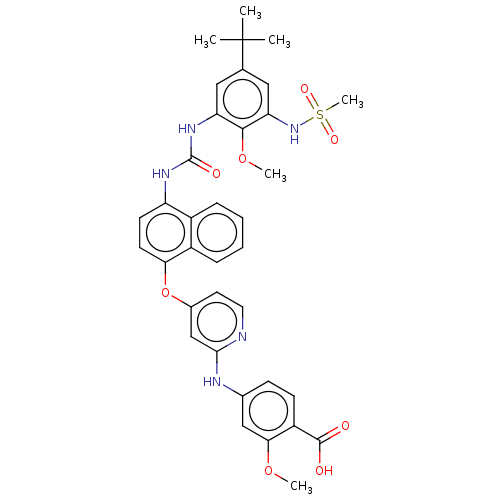 (US10125100, Example 1 | US10392346, Example 1 | US...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298376 (4-((4-((4-(3-(5-(tert-Butyl)-3-carbamoyl-2-methoxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 139 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298414 (US10125100, Example 17(t) | US10392346, Example 17...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298448 (4-[[4-[[4-[[5-tert-Butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 225 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298413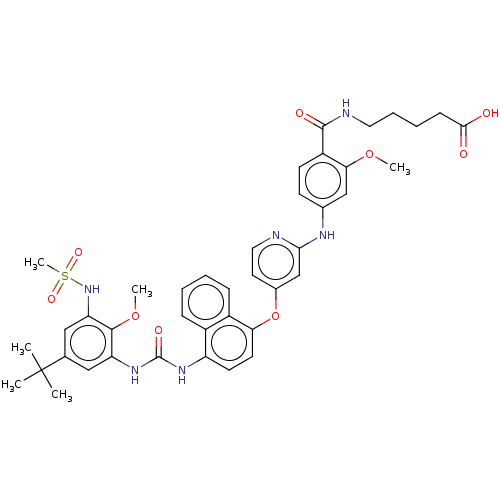 (US10125100, Example 17(s) | US10392346, Example 17...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 394 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM337692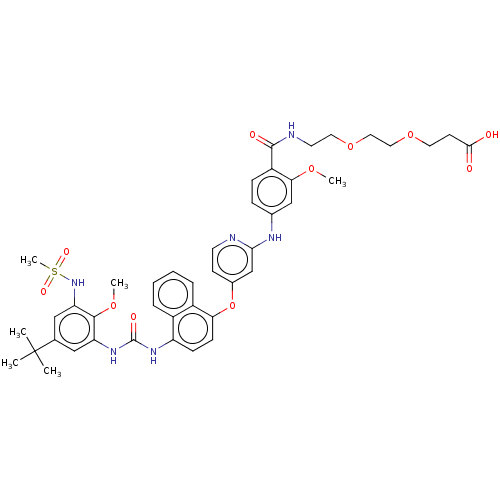 (US10392346, Example 17(x) | US10941115, Example 17...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 412 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298403 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 594 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298422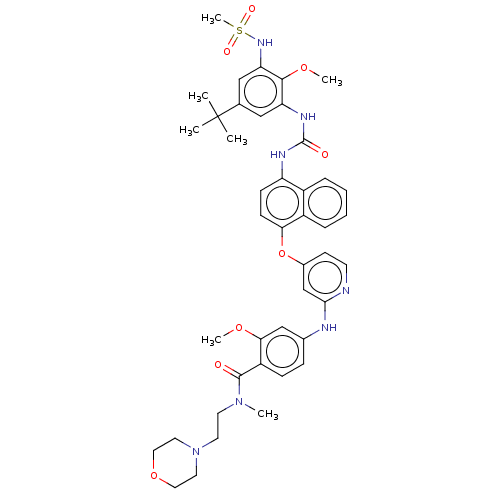 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 596 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298417 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 674 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298426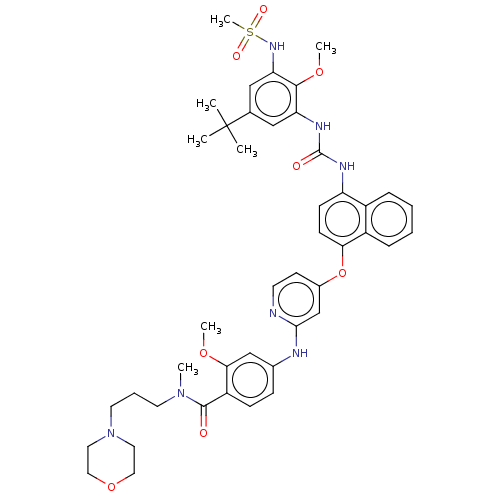 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298400 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298382 (4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298386 (N-(5-(tert-Butyl)-2-methoxy-3-(3-(4-((2-((3-methox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298380 (4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298431 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298381 (4-((4-((4-(3-(5-(tert-Butyl)-3-carbamoyl-2-methoxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298411 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298404 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298379 (4-((4-((4-(3-(5-(tert-Butyl)-3-carbamoyl-2-methoxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298390 (1-[5-tert-Butyl-3-(methanesulfonamido)-2-methoxy-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298392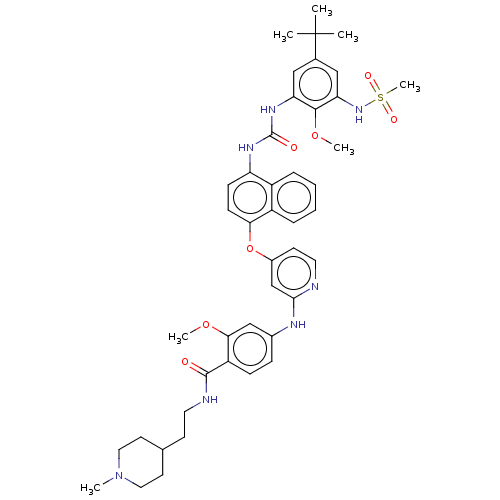 (4-[[4-[[4-[[5-tert-Butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298415 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298375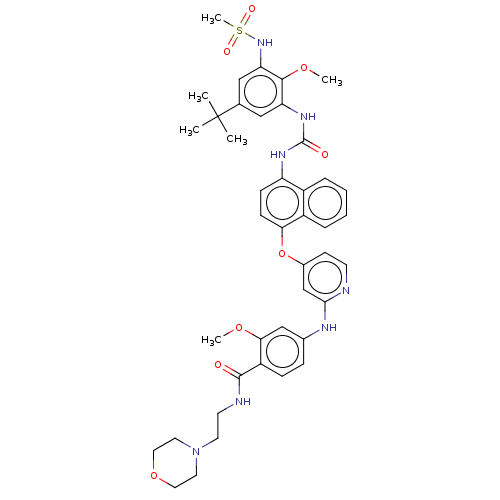 (4-((4-((4-(3-(5-(tert-Butyl)-2-methoxy-3-(methylsu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298430 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM411487 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298407 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298393 (4-[[4-[[4-[[5-tert-Butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298420 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298421 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298425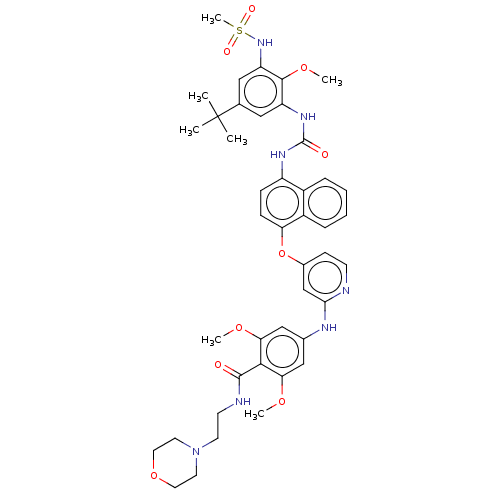 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298423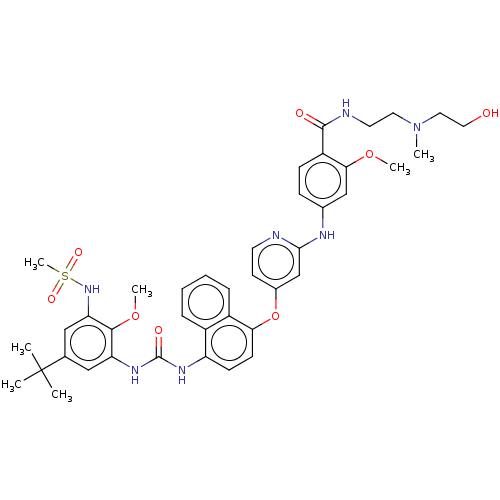 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298424 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298409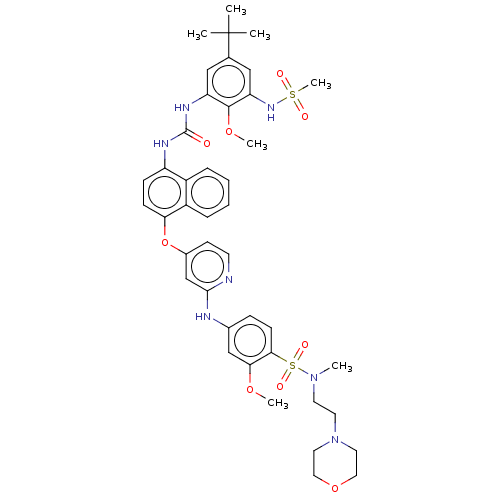 (1-[5-tert-butyl-3-(methanesulfonamido)-2-methoxy-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298391 (1-[5-tert-Butyl-3-(methanesulfonamido)-2-methoxy-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298394 (4-[[4-[[4-[[5-tert-Butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298383 (N-(5-(tert-Butyl)-3-(3-(4-((2-((3-cyano-4-(2-(2-(2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM411450 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298412 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298401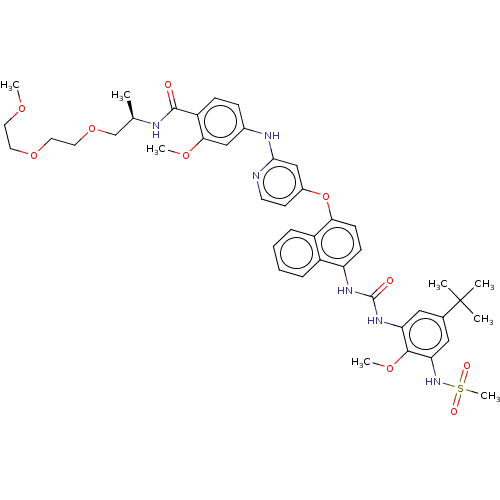 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298384 (N-(5-(tert-Butyl)-3-(3-(4-((2-((3-cyano-4-(2-morph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298402 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298429 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298396 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298410 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298385 (N-(5-(tert-Butyl)-3-(3-(4-((2-((3-cyano-4-(3-morph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298419 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298399 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM411488 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 9.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298432 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 alpha (Homo sapiens (Human)) | BDBM298408 (4-[[4-[[4-[[5-tert-butyl-3-(methanesulfonamido)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Topivert Pharma Limited US Patent | Assay Description GSK 3α Method 2: This method follows the same steps as Method 1, but utilises a shorter period of mixing of the test compound (105 minutes inste... | US Patent US10392346 (2019) BindingDB Entry DOI: 10.7270/Q2N018WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 55 total ) | Next | Last >> |