

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052125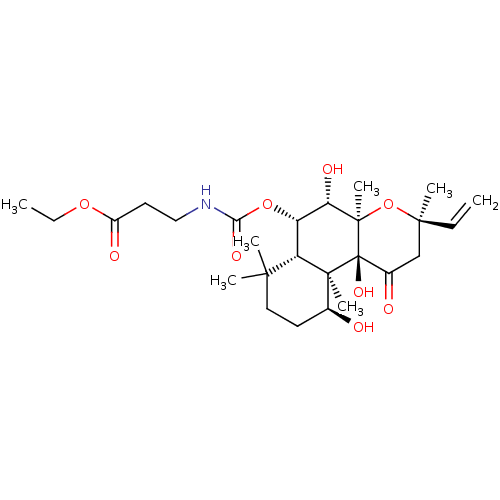 (3-((3R,4aR,5S,6S,6aS,10S,10aR,10bS)-5,10,10b-Trihy...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Ability to activate the conversion of [32P] ATP to [32P]-cyclic AMP mediated by adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052111 ((2-Methoxy-ethyl)-carbamic acid (3R,4aR,5S,6S,6aS,...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Ability to activate the conversion of [32P] ATP to [32P]-cyclic AMP mediated by adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052144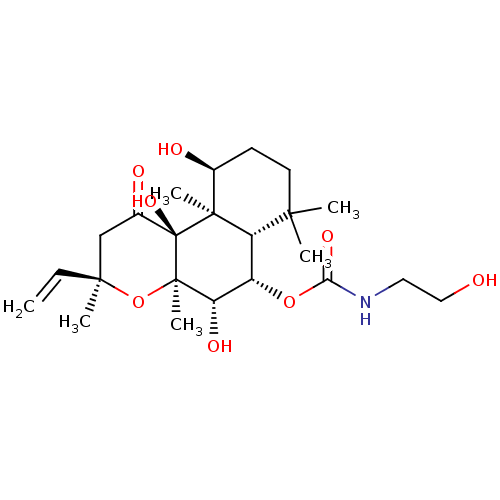 ((2-Hydroxy-ethyl)-carbamic acid (3R,4aR,5S,6S,6aS,...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Ability to activate the conversion of [32P] ATP to [32P]-cyclic AMP mediated by adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052134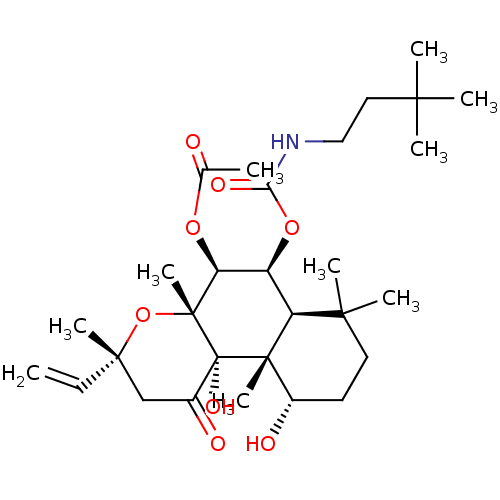 (Acetic acid (3R,4aR,5S,6S,6aS,10S,10aR,10bS)-6-(3,...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Ability to activate the conversion of [32P] ATP to [32P]-cyclic AMP mediated by adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052113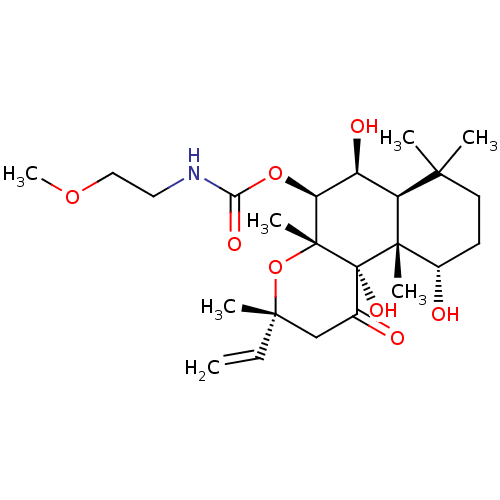 ((2-Methoxy-ethyl)-carbamic acid (3R,4aR,5S,6S,6aS,...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Ability to activate the conversion of [32P] ATP to [32P]-cyclic AMP mediated by adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052120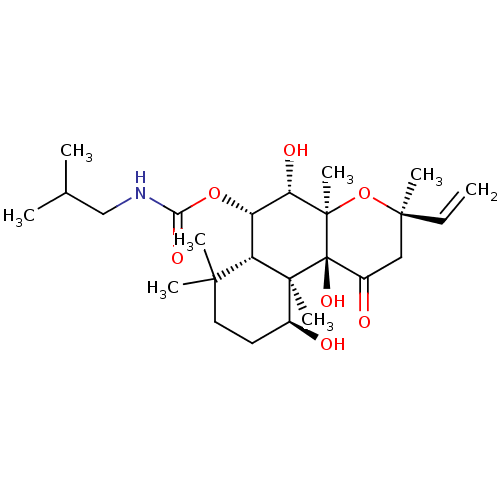 (CHEMBL419139 | Isobutyl-carbamic acid (3R,4aR,5S,6...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Ability to activate the conversion of [32P] ATP to [32P]-cyclic AMP mediated by adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052115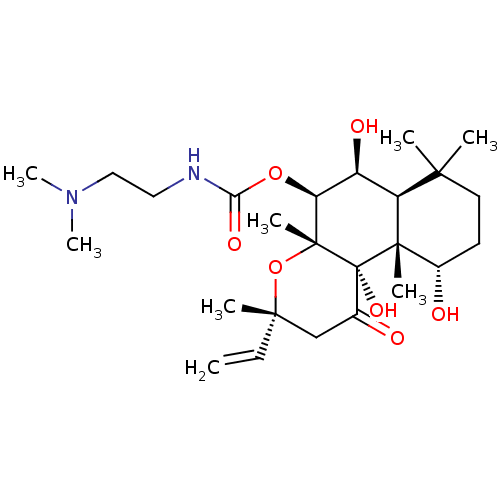 ((2-Dimethylamino-ethyl)-carbamic acid (3R,4aR,5S,6...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Ability to activate the conversion of [32P] ATP to [32P]-cyclic AMP mediated by adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052139 (Allyl-carbamic acid (3R,4aR,5S,6S,6aS,10S,10aR,10b...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Ability to activate the conversion of [32P] ATP to [32P]-cyclic AMP mediated by adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052122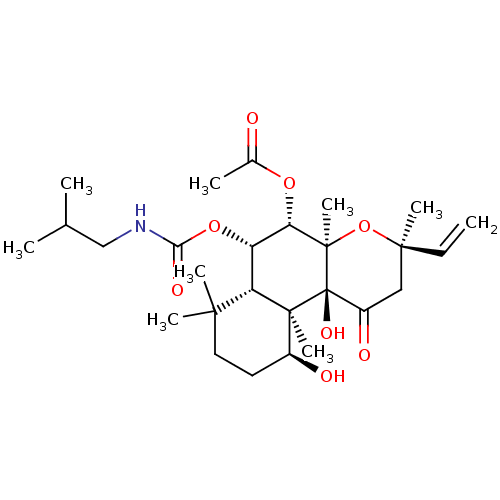 (Acetic acid (3R,4aR,5S,6S,6aS,10S,10aR,10bS)-10,10...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Ability to activate the conversion of [32P] ATP to [32P]-cyclic AMP mediated by adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052121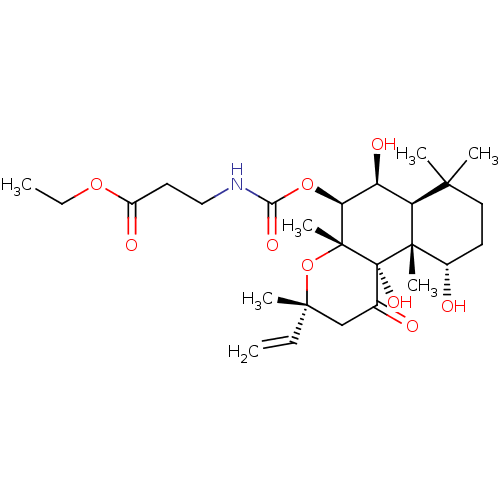 (3-((3R,4aR,5S,6S,6aS,10S,10aR,10bS)-6,10,10b-Trihy...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Ability to activate the conversion of [32P] ATP to [32P]-cyclic AMP mediated by adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052145 (CHEMBL93252 | [2-(4-Amino-phenyl)-ethyl]-carbamic ...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Ability to activate the conversion of [32P] ATP to [32P]-cyclic AMP mediated by adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052133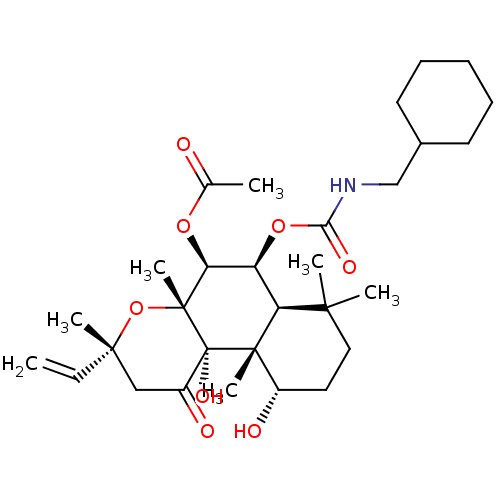 (Acetic acid (3R,4aR,5S,6S,6aS,10S,10aR,10bS)-6-cyc...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Ability to activate the conversion of [32P] ATP to [32P]-cyclic AMP mediated by adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052149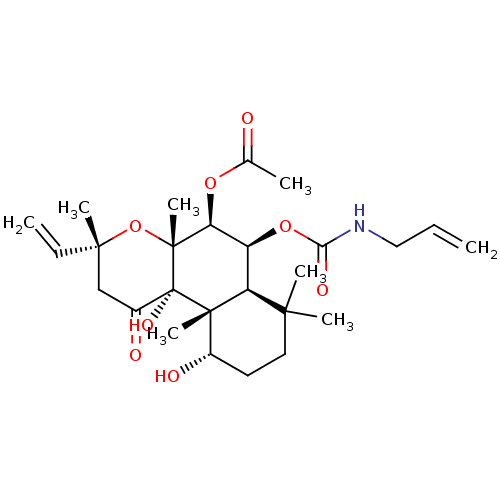 (Acetic acid (3R,4aR,5S,6S,6aS,10S,10aR,10bS)-6-all...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Ability to activate the conversion of [32P] ATP to [32P]-cyclic AMP mediated by adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052138 (Allyl-carbamic acid (3R,4aR,5S,6S,6aS,10S,10aR,10b...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Ability to activate the conversion of [32P] ATP to [32P]-cyclic AMP mediated by adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052114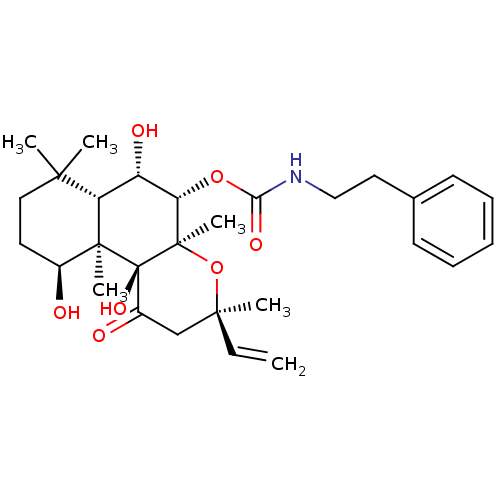 (CHEMBL93468 | Phenethyl-carbamic acid (3R,4aR,5S,6...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Ability to activate the conversion of [32P] ATP to [32P]-cyclic AMP mediated by adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052135 ((2-Dimethylamino-ethyl)-carbamic acid (3R,4aR,5S,6...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Ability to activate the conversion of [32P] ATP to [32P]-cyclic AMP mediated by adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052128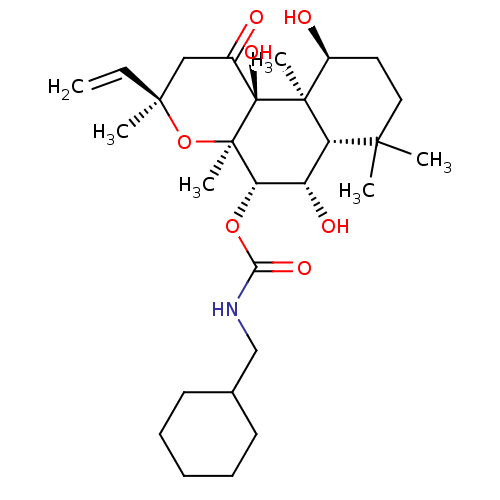 (CHEMBL92687 | Cyclohexylmethyl-carbamic acid (3R,4...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Ability to activate the conversion of [32P] ATP to [32P]-cyclic AMP mediated by adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052118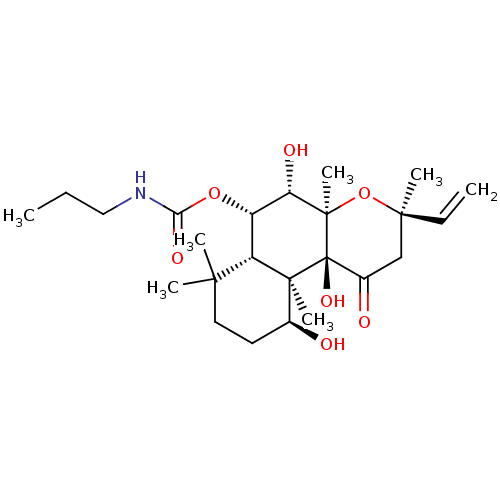 (CHEMBL330704 | Propyl-carbamic acid (3R,4aR,5S,6S,...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Ability to activate the conversion of [32P] ATP to [32P]-cyclic AMP mediated by adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052116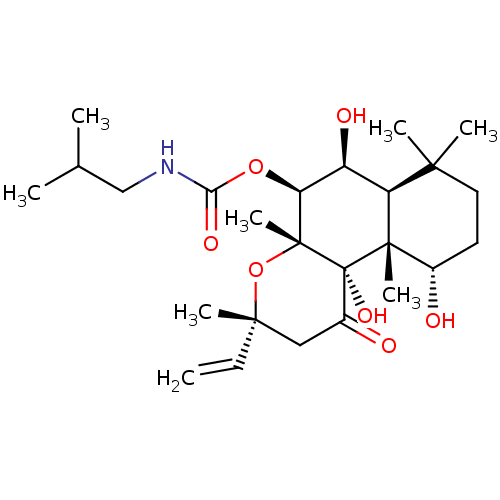 (CHEMBL93260 | Isobutyl-carbamic acid (3R,4aR,5S,6S...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Ability to activate the conversion of [32P] ATP to [32P]-cyclic AMP mediated by adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052119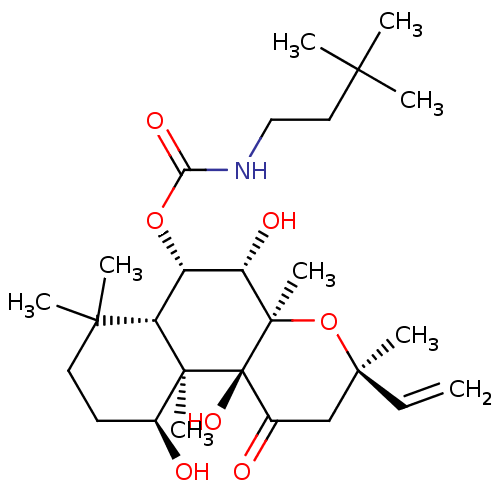 ((3,3-Dimethyl-butyl)-carbamic acid (3R,4aR,5S,6S,6...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Ability to activate the conversion of [32P] ATP to [32P]-cyclic AMP mediated by adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052136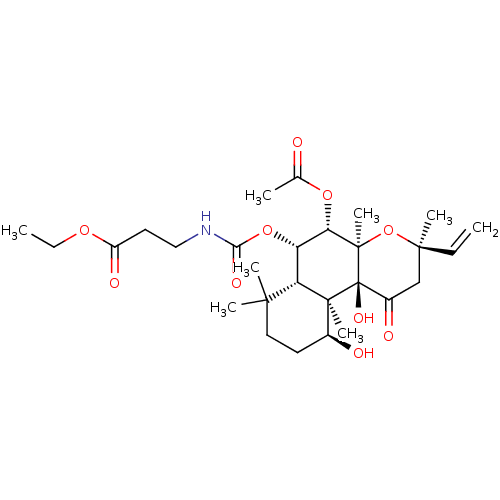 (3-((3R,4aR,5S,6S,6aS,10S,10aR,10bS)-5-Acetoxy-10,1...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Ability to activate the conversion of [32P] ATP to [32P]-cyclic AMP mediated by adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052112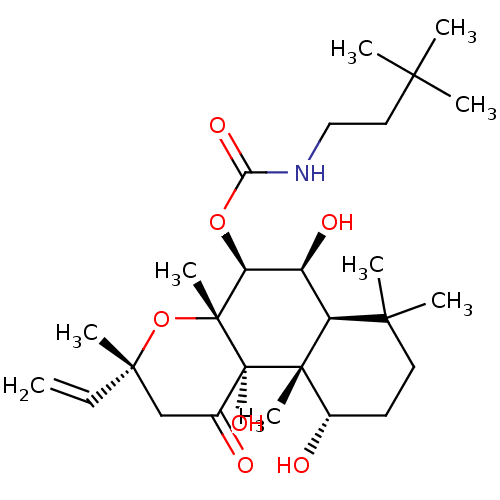 ((3,3-Dimethyl-butyl)-carbamic acid (3R,4aR,5S,6S,6...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Ability to activate the conversion of [32P] ATP to [32P]-cyclic AMP mediated by adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1 (Homo sapiens (Human)) | BDBM50052127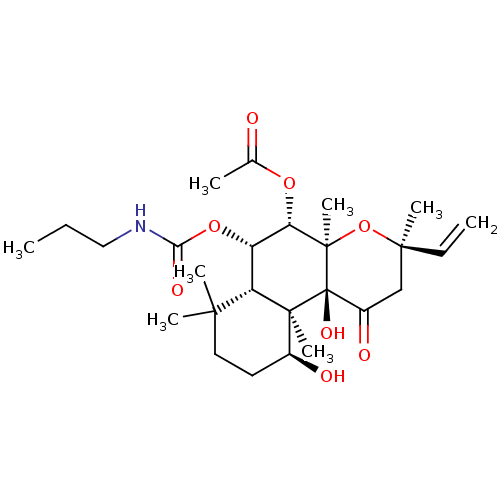 (Acetic acid (3R,4aR,5S,6S,6aS,10S,10aR,10bS)-10,10...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Ability to activate the conversion of [32P] ATP to [32P]-cyclic AMP mediated by adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||