

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Integrase (Human immunodeficiency virus 1) | BDBM50145956 ((Z)-4-(3-{4-[3-(3-Carboxy-3-hydroxy-acryloyl)-phen...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 integrase catalytic activity in terms of inhibition of 3'-processing | J Med Chem 47: 2561-73 (2004) Article DOI: 10.1021/jm030559k BindingDB Entry DOI: 10.7270/Q29P3136 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50145950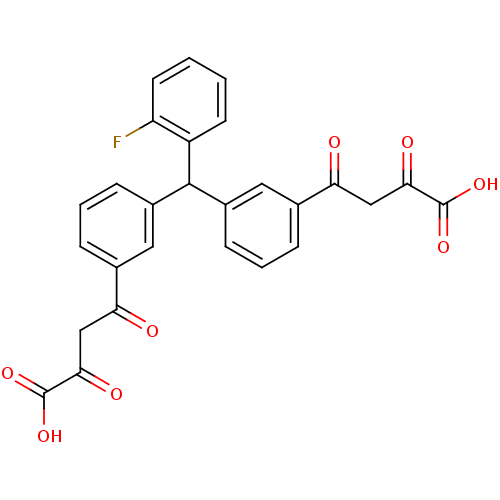 ((Z)-4-{3-[[3-(3-Carboxy-3-hydroxy-acryloyl)-phenyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 integrase catalytic activity in terms of inhibition of 3'-processing | J Med Chem 47: 2561-73 (2004) Article DOI: 10.1021/jm030559k BindingDB Entry DOI: 10.7270/Q29P3136 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50145958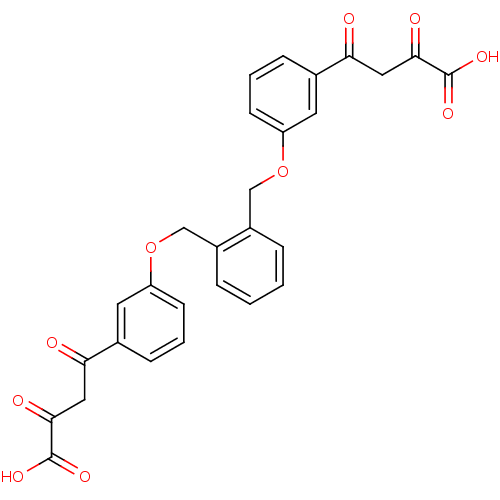 ((Z)-4-(3-{2-[3-(3-Carboxy-3-hydroxy-acryloyl)-phen...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 integrase catalytic activity in terms of inhibition of 3'-processing | J Med Chem 47: 2561-73 (2004) Article DOI: 10.1021/jm030559k BindingDB Entry DOI: 10.7270/Q29P3136 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50095515 (4-[3-(2-Fluoro-benzyl)-phenyl]-2,4-dioxo-butyric a...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 integrase catalytic activity in terms of inhibition of 3'-processing | J Med Chem 47: 2561-73 (2004) Article DOI: 10.1021/jm030559k BindingDB Entry DOI: 10.7270/Q29P3136 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50145953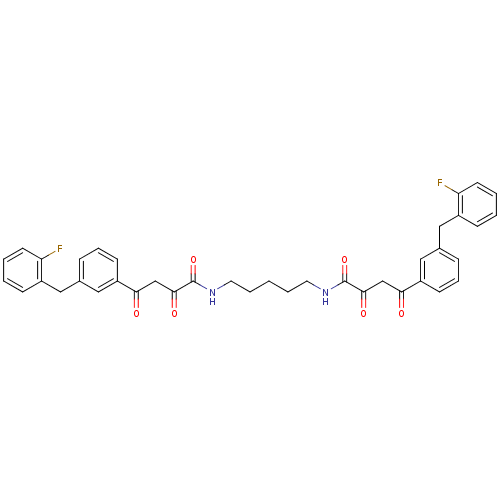 ((Z)-4-[3-(2-Fluoro-benzyl)-phenyl]-2-hydroxy-4-oxo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 integrase catalytic activity in terms of inhibition of 3'-processing | J Med Chem 47: 2561-73 (2004) Article DOI: 10.1021/jm030559k BindingDB Entry DOI: 10.7270/Q29P3136 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50145954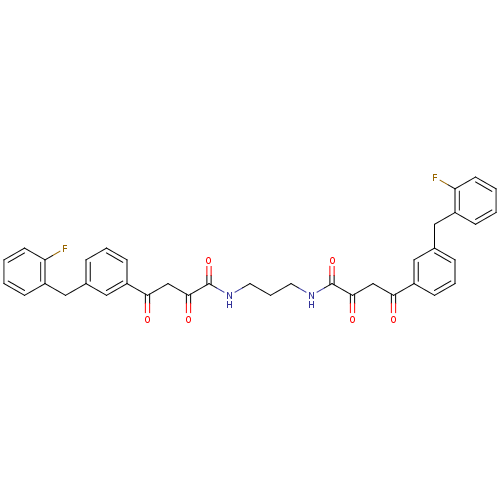 ((Z)-4-[3-(2-Fluoro-benzyl)-phenyl]-2-hydroxy-4-oxo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 integrase catalytic activity in terms of inhibition of 3'-processing | J Med Chem 47: 2561-73 (2004) Article DOI: 10.1021/jm030559k BindingDB Entry DOI: 10.7270/Q29P3136 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50145948 ((Z)-4-[3-(2-Fluoro-benzyl)-phenyl]-2-hydroxy-4-oxo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 integrase catalytic activity in terms of inhibition of 3'-processing | J Med Chem 47: 2561-73 (2004) Article DOI: 10.1021/jm030559k BindingDB Entry DOI: 10.7270/Q29P3136 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50145957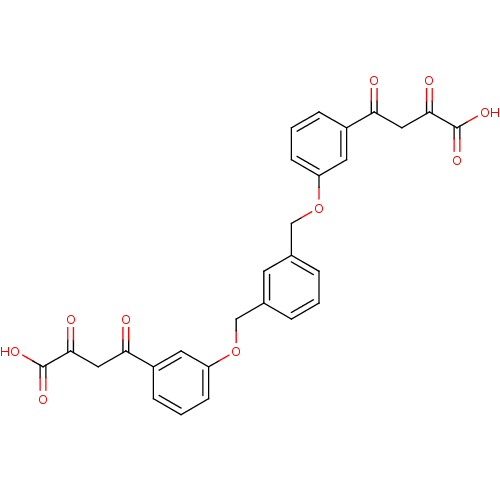 ((Z)-4-(3-{3-[3-(3-Carboxy-3-hydroxy-acryloyl)-phen...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 integrase catalytic activity in terms of inhibition of 3'-processing | J Med Chem 47: 2561-73 (2004) Article DOI: 10.1021/jm030559k BindingDB Entry DOI: 10.7270/Q29P3136 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM23399 (4-{1-[(4-fluorophenyl)methyl]-1H-pyrrol-2-yl}-2,4-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 integrase catalytic activity in terms of inhibition of 3'-processing | J Med Chem 47: 2561-73 (2004) Article DOI: 10.1021/jm030559k BindingDB Entry DOI: 10.7270/Q29P3136 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50145955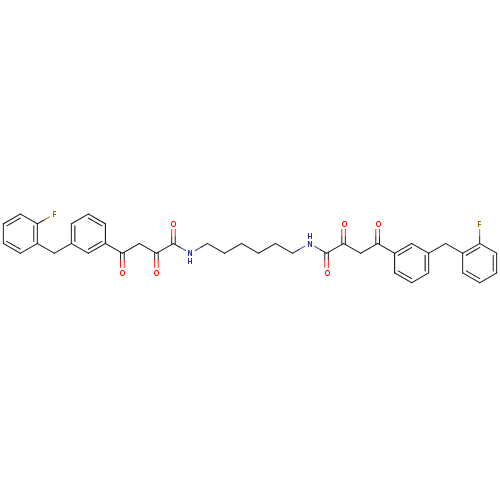 ((Z)-4-[3-(2-Fluoro-benzyl)-phenyl]-2-hydroxy-4-oxo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica Curated by ChEMBL | Assay Description Evaluated for inhibition of HIV-1 integrase catalytic activity in terms of inhibition of 3'-processing | J Med Chem 47: 2561-73 (2004) Article DOI: 10.1021/jm030559k BindingDB Entry DOI: 10.7270/Q29P3136 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||