

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G-protein coupled receptor 35 (Homo sapiens (Human)) | BDBM50436001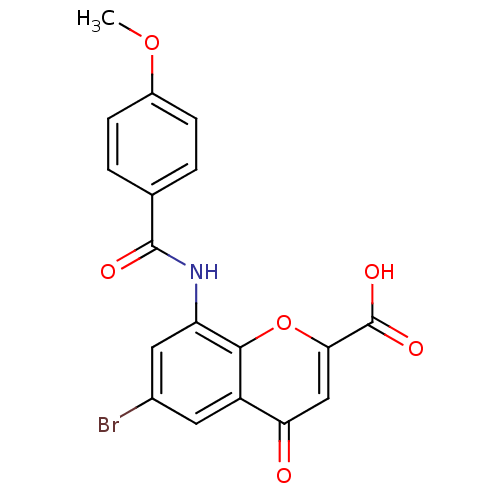 (CHEMBL2392174 | CHEMBL2425818) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Dalian Institute of Chemical Physics Curated by ChEMBL | Assay Description Agonist activity at human GPR35 expressed in CHO-K1 cells after 90 mins by beta-arrestin 2 recruitment assay | J Med Chem 60: 362-372 (2017) Article DOI: 10.1021/acs.jmedchem.6b01431 BindingDB Entry DOI: 10.7270/Q2J67KC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 35 (Homo sapiens (Human)) | BDBM38549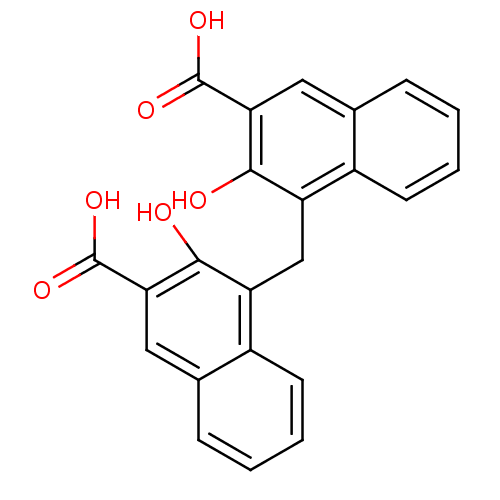 (2-[2-[4-[(4-chlorophenyl)-phenyl-methyl]piperazino...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
Dalian Institute of Chemical Physics Curated by ChEMBL | Assay Description Agonist activity at human GPR35 expressed in CHO-K1 cells after 90 mins by beta-arrestin 2 recruitment assay | J Med Chem 60: 362-372 (2017) Article DOI: 10.1021/acs.jmedchem.6b01431 BindingDB Entry DOI: 10.7270/Q2J67KC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 35 (Homo sapiens (Human)) | BDBM50259887 (CHEMBL3277775) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Dalian Institute of Chemical Physics Curated by ChEMBL | Assay Description Agonist activity at human GPR35 expressed in CHO-K1 cells after 90 mins by beta-arrestin 2 recruitment assay | J Med Chem 60: 362-372 (2017) Article DOI: 10.1021/acs.jmedchem.6b01431 BindingDB Entry DOI: 10.7270/Q2J67KC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 35 (Homo sapiens (Human)) | BDBM35525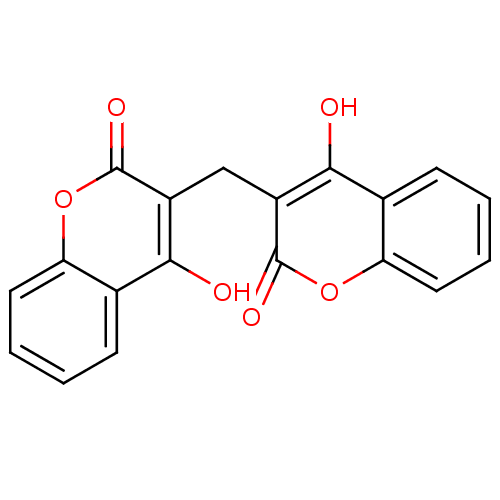 (3,3''''-methylenebis(4-hydroxy-coumarin | 3,3''''-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a |
Dalian Institute of Chemical Physics Curated by ChEMBL | Assay Description Agonist activity at human GPR35 expressed in CHO-K1 cells after 90 mins by beta-arrestin 2 recruitment assay | J Med Chem 60: 362-372 (2017) Article DOI: 10.1021/acs.jmedchem.6b01431 BindingDB Entry DOI: 10.7270/Q2J67KC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 35 (Homo sapiens (Human)) | BDBM14363 (3-(2-propoxyphenyl)-2,4,7,8,9-pentazabicyclo[4.3.0...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a |
Dalian Institute of Chemical Physics Curated by ChEMBL | Assay Description Agonist activity at human GPR35 expressed in CHO-K1 cells after 90 mins by beta-arrestin 2 recruitment assay | J Med Chem 60: 362-372 (2017) Article DOI: 10.1021/acs.jmedchem.6b01431 BindingDB Entry DOI: 10.7270/Q2J67KC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 35 (Homo sapiens (Human)) | BDBM50440033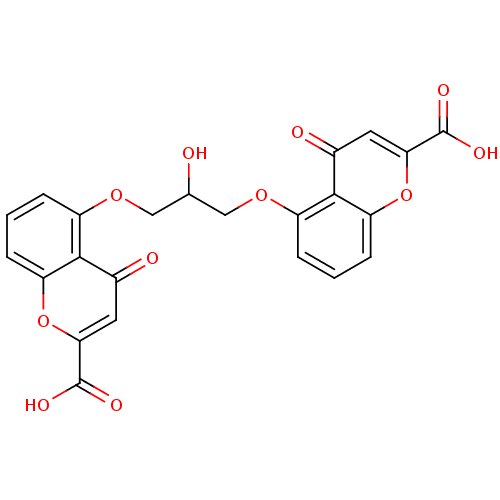 (CROMOLYN | Crolom | Cromoglicic Acid) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a |
Dalian Institute of Chemical Physics Curated by ChEMBL | Assay Description Agonist activity at human GPR35 expressed in CHO-K1 cells after 90 mins by beta-arrestin 2 recruitment assay | J Med Chem 60: 362-372 (2017) Article DOI: 10.1021/acs.jmedchem.6b01431 BindingDB Entry DOI: 10.7270/Q2J67KC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 35 (Homo sapiens (Human)) | BDBM50357187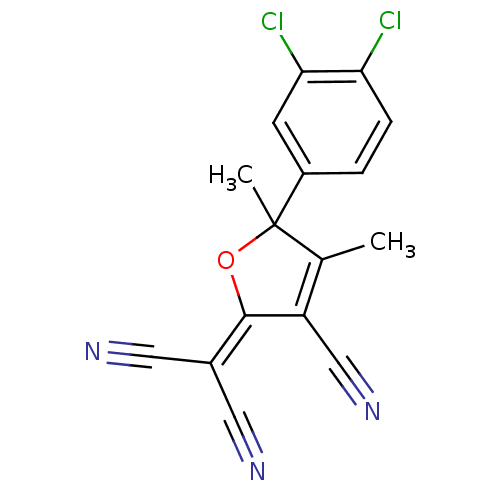 (CHEMBL1914576) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a |
Dalian Institute of Chemical Physics Curated by ChEMBL | Assay Description Agonist activity at human GPR35 expressed in CHO-K1 cells after 90 mins by beta-arrestin 2 recruitment assay | J Med Chem 60: 362-372 (2017) Article DOI: 10.1021/acs.jmedchem.6b01431 BindingDB Entry DOI: 10.7270/Q2J67KC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 35 (Homo sapiens (Human)) | BDBM50259889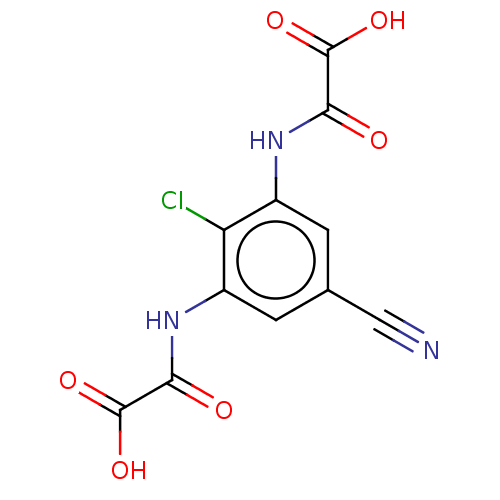 (Lodoxamide) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a |
Dalian Institute of Chemical Physics Curated by ChEMBL | Assay Description Agonist activity at human GPR35 expressed in CHO-K1 cells after 90 mins by beta-arrestin 2 recruitment assay | J Med Chem 60: 362-372 (2017) Article DOI: 10.1021/acs.jmedchem.6b01431 BindingDB Entry DOI: 10.7270/Q2J67KC8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| G-protein coupled receptor 35 (Homo sapiens (Human)) | BDBM50233945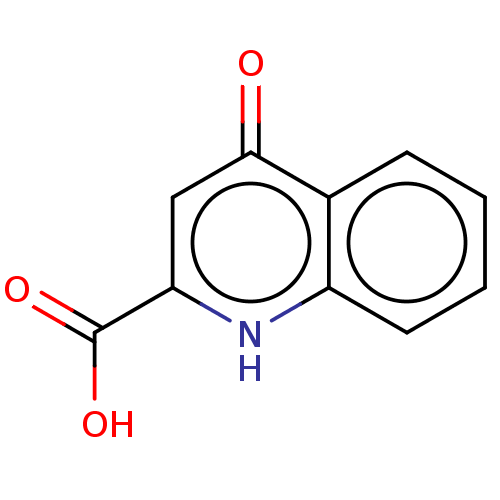 (CHEBI:18344 | Kynurenic Acid | TRANSTORINE) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.17E+5 | n/a | n/a | n/a | n/a |
Dalian Institute of Chemical Physics Curated by ChEMBL | Assay Description Agonist activity at human GPR35 expressed in CHO-K1 cells after 90 mins by beta-arrestin 2 recruitment assay | J Med Chem 60: 362-372 (2017) Article DOI: 10.1021/acs.jmedchem.6b01431 BindingDB Entry DOI: 10.7270/Q2J67KC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 35 (Homo sapiens (Human)) | BDBM357857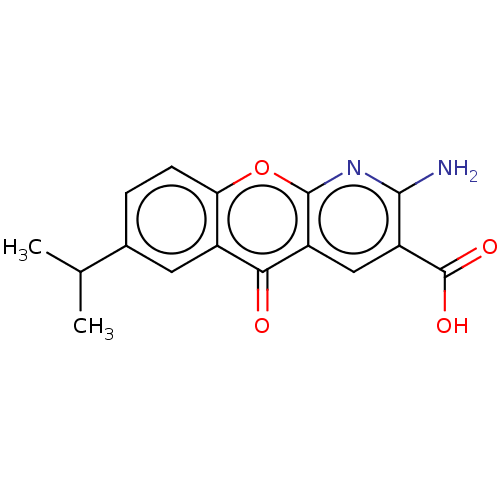 (Aphthasol | US10214536, Amlexanox) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a |
Dalian Institute of Chemical Physics Curated by ChEMBL | Assay Description Agonist activity at human GPR35 expressed in CHO-K1 cells after 90 mins by beta-arrestin 2 recruitment assay | J Med Chem 60: 362-372 (2017) Article DOI: 10.1021/acs.jmedchem.6b01431 BindingDB Entry DOI: 10.7270/Q2J67KC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||