

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM578799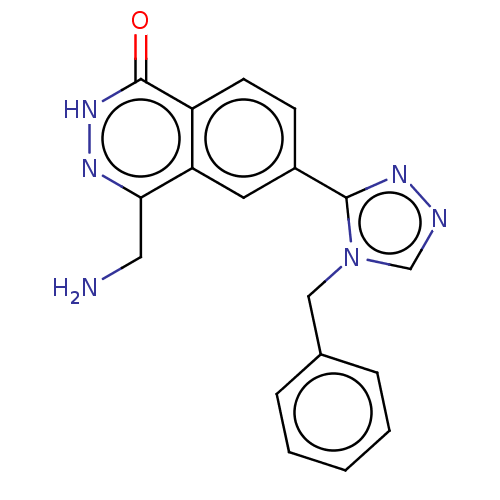 (4-(aminomethyl)-6-(4-benzyl-4H-1,2,4-triazol-3- yl...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu... | Citation and Details BindingDB Entry DOI: 10.7270/Q23F4SWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM578810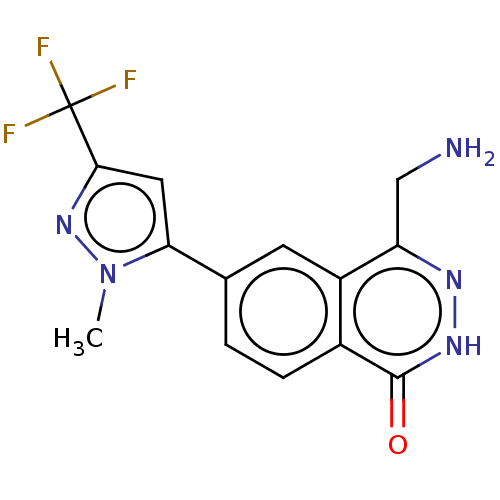 (4-(aminomethyl)-6-(1-methyl-3-(trifluoromethyl)-1H...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu... | Citation and Details BindingDB Entry DOI: 10.7270/Q23F4SWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM578828 (4-(aminomethyl)-6-(1-methyl-1H-pyrrolo[2,3-b]pyrid...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu... | Citation and Details BindingDB Entry DOI: 10.7270/Q23F4SWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM578954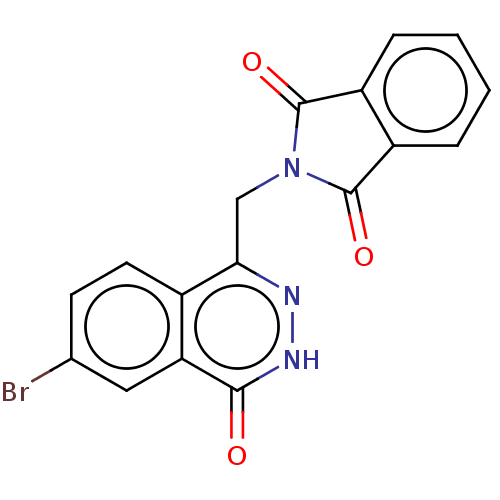 (US11479551, Example 13-1 | US11479551, Example 6-2...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu... | Citation and Details BindingDB Entry DOI: 10.7270/Q2V128N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM578799 (4-(aminomethyl)-6-(4-benzyl-4H-1,2,4-triazol-3- yl...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu... | Citation and Details BindingDB Entry DOI: 10.7270/Q2V128N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM578810 (4-(aminomethyl)-6-(1-methyl-3-(trifluoromethyl)-1H...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu... | Citation and Details BindingDB Entry DOI: 10.7270/Q2V128N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM578828 (4-(aminomethyl)-6-(1-methyl-1H-pyrrolo[2,3-b]pyrid...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu... | Citation and Details BindingDB Entry DOI: 10.7270/Q2V128N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM578954 (US11479551, Example 13-1 | US11479551, Example 6-2...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu... | Citation and Details BindingDB Entry DOI: 10.7270/Q23F4SWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50462572 (CHEMBL4249337) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Prelude Therapeutics Curated by ChEMBL | Assay Description Inhibition of full-length human N-terminal FLAG-tagged PRMT5/full length N-terminal His6-tagged MEP50 (unknown origin) expressed in baculovirus infec... | Bioorg Med Chem Lett 29: 1264-1269 (2019) Article DOI: 10.1016/j.bmcl.2019.03.042 BindingDB Entry DOI: 10.7270/Q2ZC869N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50523629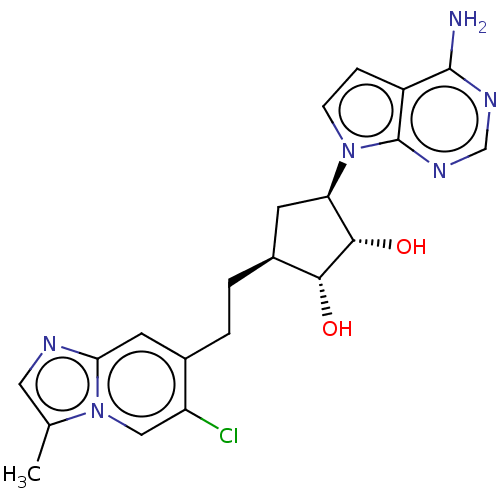 (CHEMBL4564327) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Prelude Therapeutics Curated by ChEMBL | Assay Description Inhibition of full-length human N-terminal FLAG-tagged PRMT5/full length N-terminal His6-tagged MEP50 (unknown origin) expressed in baculovirus infec... | Bioorg Med Chem Lett 29: 1264-1269 (2019) Article DOI: 10.1016/j.bmcl.2019.03.042 BindingDB Entry DOI: 10.7270/Q2ZC869N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM533464 ((E)-7-((2R,3R,4S,5S)-5-((1R)-1-(3,4- dichlorocyclo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were solubilized, and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (50 mM Tris-HCl, pH 8.5... | Citation and Details BindingDB Entry DOI: 10.7270/Q25142D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM533479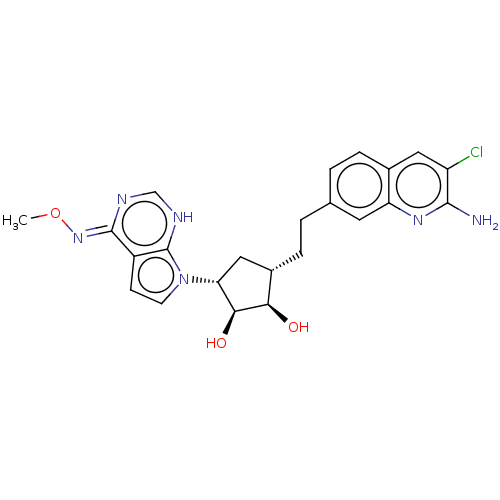 ((Z)-7-((1R,2S,3R,4S)-4-(2-(2-amino- 3-chloroquinol...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were solubilized, and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (50 mM Tris-HCl, pH 8.5... | Citation and Details BindingDB Entry DOI: 10.7270/Q25142D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50523642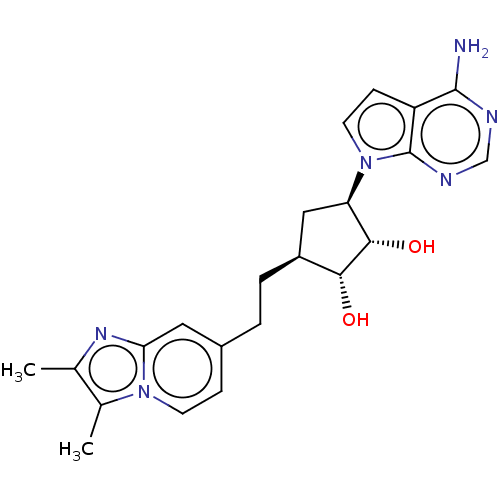 (CHEMBL4539612) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Prelude Therapeutics Curated by ChEMBL | Assay Description Inhibition of full-length human N-terminal FLAG-tagged PRMT5/full length N-terminal His6-tagged MEP50 (unknown origin) expressed in baculovirus infec... | Bioorg Med Chem Lett 29: 1264-1269 (2019) Article DOI: 10.1016/j.bmcl.2019.03.042 BindingDB Entry DOI: 10.7270/Q2ZC869N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM515298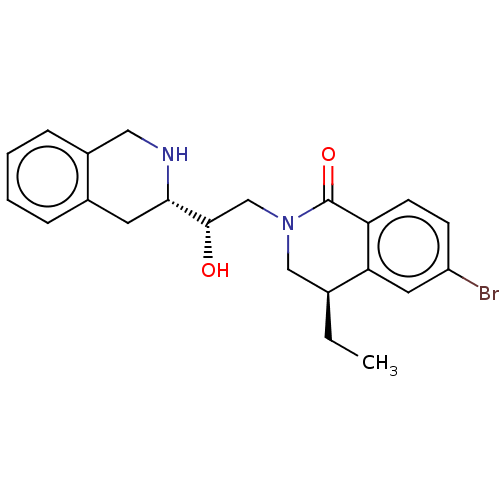 (US11098059, Example 2 | US11098059, Example 6) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ... | Citation and Details BindingDB Entry DOI: 10.7270/Q26D5X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM515310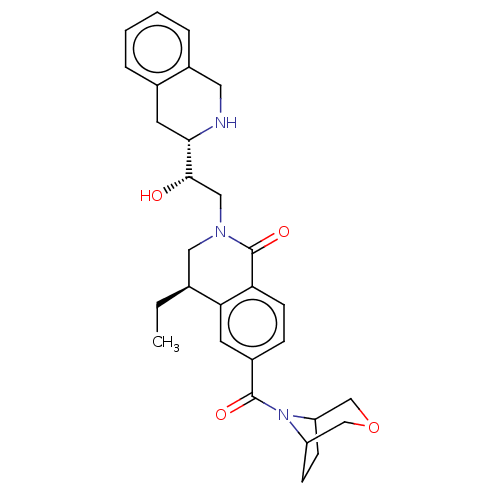 (US11098059, Example 14) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ... | Citation and Details BindingDB Entry DOI: 10.7270/Q26D5X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50523646 (CHEMBL4541714) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Prelude Therapeutics Curated by ChEMBL | Assay Description Inhibition of full-length human N-terminal FLAG-tagged PRMT5/full length N-terminal His6-tagged MEP50 (unknown origin) expressed in baculovirus infec... | Bioorg Med Chem Lett 29: 1264-1269 (2019) Article DOI: 10.1016/j.bmcl.2019.03.042 BindingDB Entry DOI: 10.7270/Q2ZC869N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM515307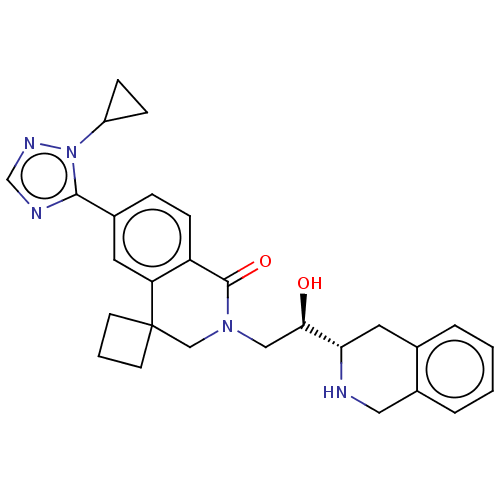 (6'-(1-cyclopropyl-1H-1,2,4- triazol-5-yl)-2'-{(2R)...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ... | Citation and Details BindingDB Entry DOI: 10.7270/Q26D5X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM533440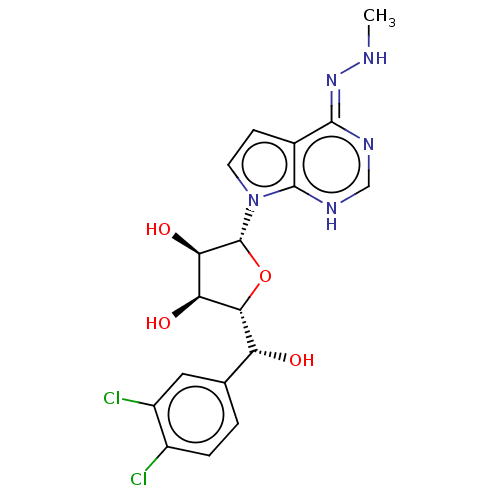 ((2R,3S,4R,5R)-2-((R)-(3,4- dichlorophenyl)(hydroxy...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were solubilized, and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (50 mM Tris-HCl, pH 8.5... | Citation and Details BindingDB Entry DOI: 10.7270/Q25142D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM515299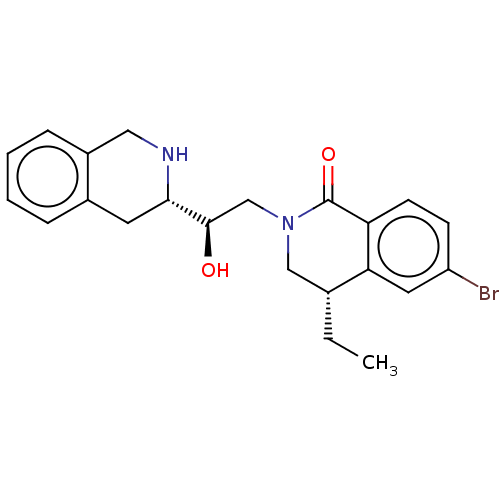 (US11098059, Example 3 | US11098059, Example 7) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ... | Citation and Details BindingDB Entry DOI: 10.7270/Q26D5X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50523655 (CHEMBL4577464) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Prelude Therapeutics Curated by ChEMBL | Assay Description Inhibition of full-length human N-terminal FLAG-tagged PRMT5/full length N-terminal His6-tagged MEP50 (unknown origin) expressed in baculovirus infec... | Bioorg Med Chem Lett 29: 1264-1269 (2019) Article DOI: 10.1016/j.bmcl.2019.03.042 BindingDB Entry DOI: 10.7270/Q2ZC869N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM533482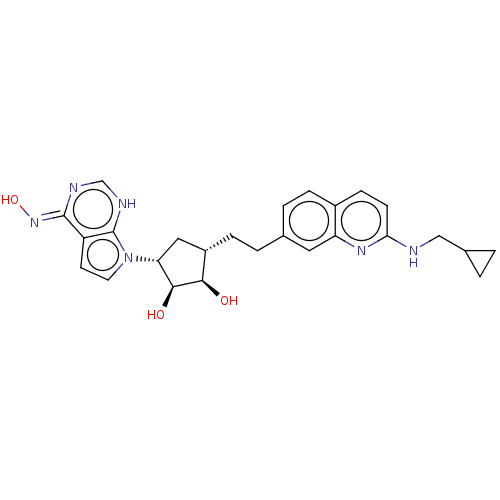 ((Z)-7-((1R,2S,3R,4S)-4-(2-(2- ((cyclopropylmethyl)...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were solubilized, and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (50 mM Tris-HCl, pH 8.5... | Citation and Details BindingDB Entry DOI: 10.7270/Q25142D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM515323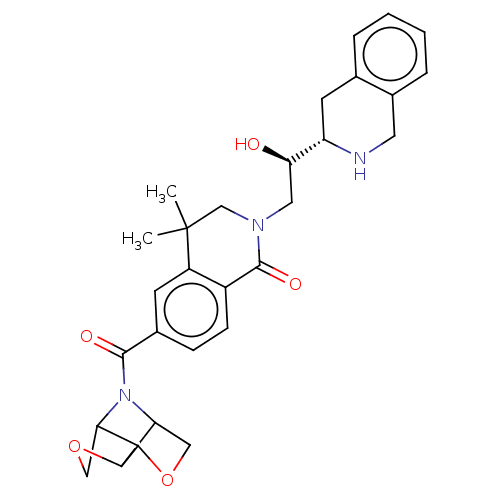 (6-(hexahydro-3,6- epiminofuro[3,2-b]furan- 7-carbo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ... | Citation and Details BindingDB Entry DOI: 10.7270/Q26D5X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM515309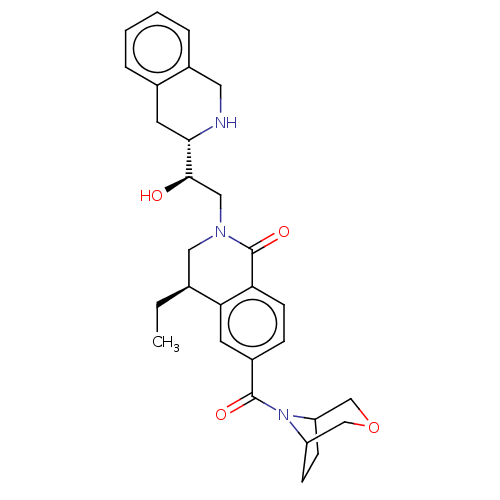 (US11098059, Example 13) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ... | Citation and Details BindingDB Entry DOI: 10.7270/Q26D5X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM533414 (7-((2R,3R,4S,5R)-5-((R)-(3,4- dichlorophenyl)(hydr...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were solubilized, and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (50 mM Tris-HCl, pH 8.5... | Citation and Details BindingDB Entry DOI: 10.7270/Q25142D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM515306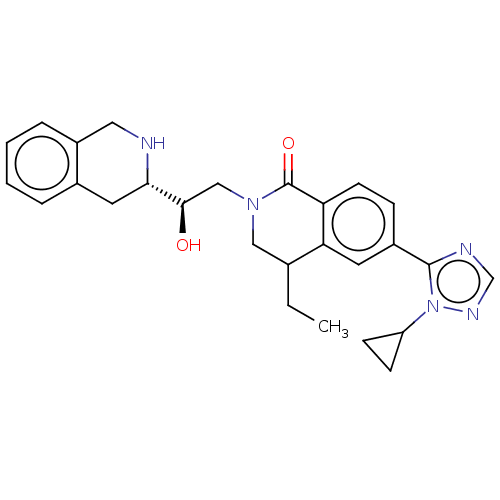 (6-(1-cyclopropyl-1H-1,2,4- triazol-5-yl)-4-ethyl-2...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ... | Citation and Details BindingDB Entry DOI: 10.7270/Q26D5X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50523643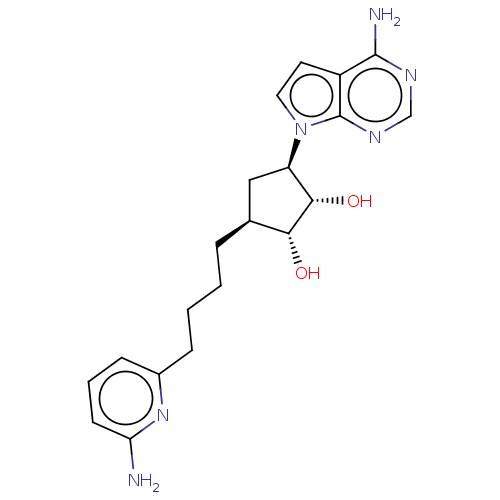 (CHEMBL4454890) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Prelude Therapeutics Curated by ChEMBL | Assay Description Inhibition of full-length human N-terminal FLAG-tagged PRMT5/full length N-terminal His6-tagged MEP50 (unknown origin) expressed in baculovirus infec... | Bioorg Med Chem Lett 29: 1264-1269 (2019) Article DOI: 10.1016/j.bmcl.2019.03.042 BindingDB Entry DOI: 10.7270/Q2ZC869N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM515321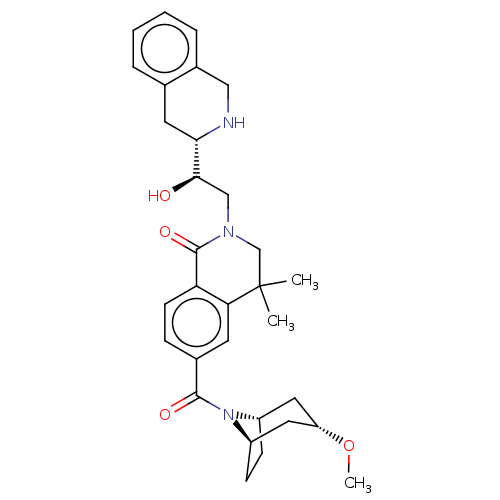 (2-((R)-2-hydroxy-2-((S)- 1,2,3,4- tetrahydroisoqui...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ... | Citation and Details BindingDB Entry DOI: 10.7270/Q26D5X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM515305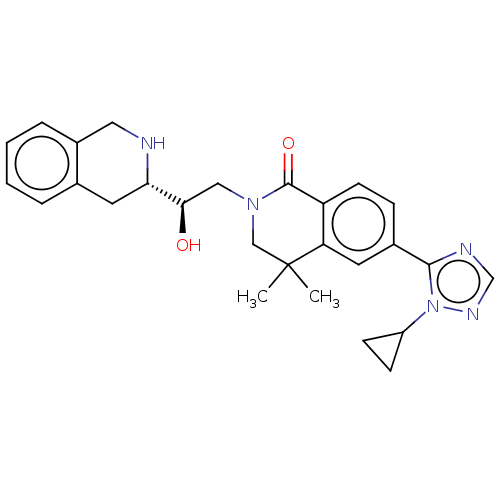 (6-(1-cyclopropyl-1H-1,2,4-triazol-5-yl)-2-{(2R)-2-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ... | Citation and Details BindingDB Entry DOI: 10.7270/Q26D5X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM578895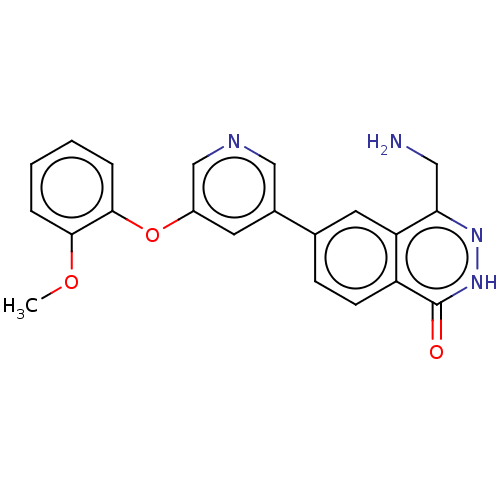 (4-(aminomethyl)-6-(5-(2- methoxyphenoxy)pyridin-3-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu... | Citation and Details BindingDB Entry DOI: 10.7270/Q23F4SWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM578831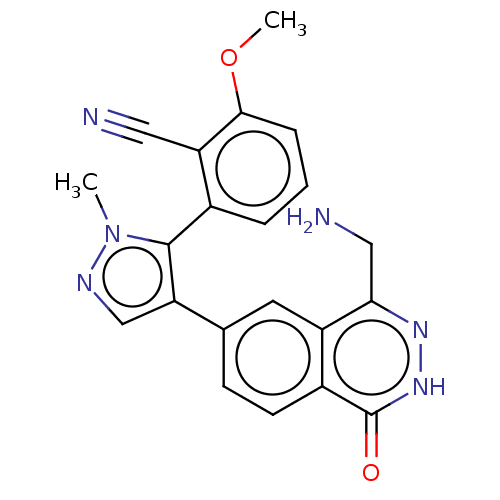 (2-(4-(4-(aminomethyl)-1-oxo-1,2- dihydrophthalazin...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu... | Citation and Details BindingDB Entry DOI: 10.7270/Q23F4SWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM578829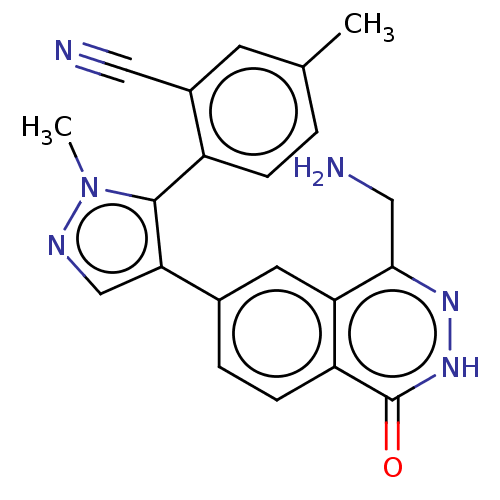 (2-(4-(4-(aminomethyl)-1-oxo-1,2- dihydrophthalazin...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu... | Citation and Details BindingDB Entry DOI: 10.7270/Q23F4SWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM578825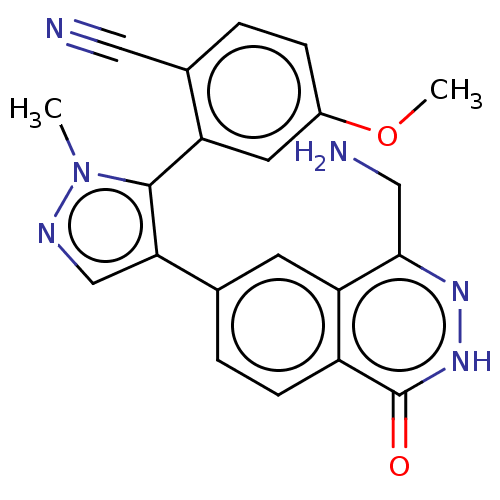 (2-(4-(4-(aminomethyl)-1-oxo-1,2- dihydrophthalazin...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu... | Citation and Details BindingDB Entry DOI: 10.7270/Q23F4SWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM578823 (2-(4-(4-(aminomethyl)-1-oxo-1,2- dihydrophthalazin...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu... | Citation and Details BindingDB Entry DOI: 10.7270/Q23F4SWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM578821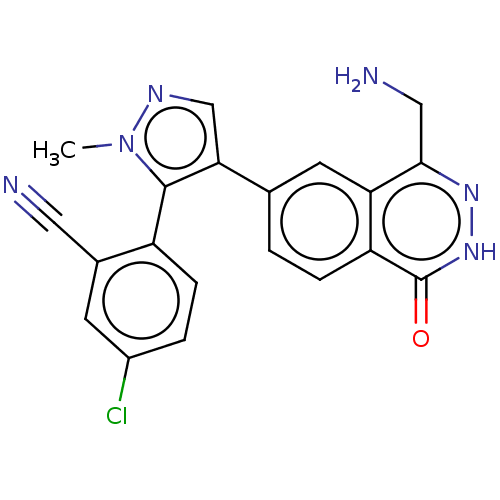 (2-(4-(4-(aminomethyl)-1-oxo-1,2- dihydrophthalazin...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu... | Citation and Details BindingDB Entry DOI: 10.7270/Q23F4SWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM578819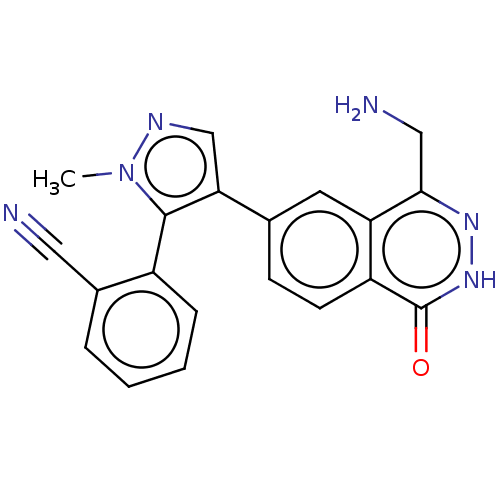 (2-(4-(4-(aminomethyl)-1-oxo-1,2- dihydrophthalazin...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu... | Citation and Details BindingDB Entry DOI: 10.7270/Q23F4SWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM435525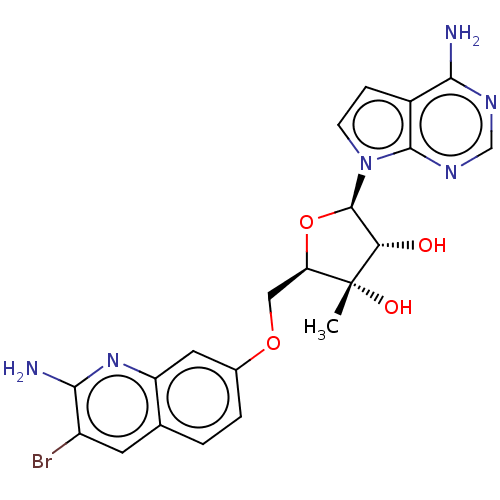 ((2R,3S,4R,5R)-2-(((2-amino-3- bromoquinolin-7-yl)o...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (50 mM Tris-HCl, pH 8.5,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2N58QJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM515319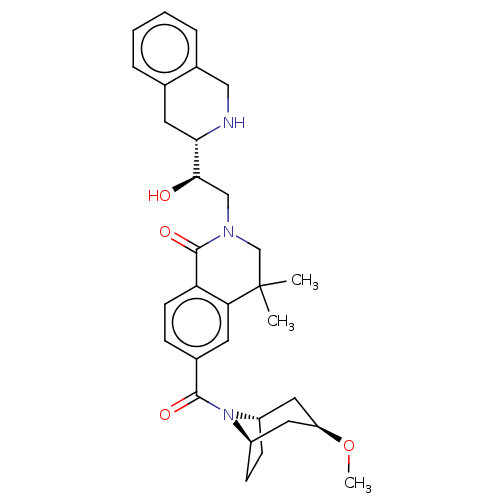 (US11098059, Example 23) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ... | Citation and Details BindingDB Entry DOI: 10.7270/Q26D5X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM578831 (2-(4-(4-(aminomethyl)-1-oxo-1,2- dihydrophthalazin...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu... | Citation and Details BindingDB Entry DOI: 10.7270/Q2V128N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM578895 (4-(aminomethyl)-6-(5-(2- methoxyphenoxy)pyridin-3-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu... | Citation and Details BindingDB Entry DOI: 10.7270/Q2V128N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM435525 ((2R,3S,4R,5R)-2-(((2-amino-3- bromoquinolin-7-yl)o...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Prelude Therapeutics Incorporated US Patent | Assay Description Compounds were solubilized and 3-fold diluted in 100% DMSO. These diluted compounds were further diluted in the assay buffer (50 mM Tris-HCl, pH 8.5,... | US Patent US10570140 (2020) BindingDB Entry DOI: 10.7270/Q2WM1GTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM578829 (2-(4-(4-(aminomethyl)-1-oxo-1,2- dihydrophthalazin...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu... | Citation and Details BindingDB Entry DOI: 10.7270/Q2V128N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM578819 (2-(4-(4-(aminomethyl)-1-oxo-1,2- dihydrophthalazin...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu... | Citation and Details BindingDB Entry DOI: 10.7270/Q2V128N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM578821 (2-(4-(4-(aminomethyl)-1-oxo-1,2- dihydrophthalazin...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu... | Citation and Details BindingDB Entry DOI: 10.7270/Q2V128N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM578823 (2-(4-(4-(aminomethyl)-1-oxo-1,2- dihydrophthalazin...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu... | Citation and Details BindingDB Entry DOI: 10.7270/Q2V128N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM578825 (2-(4-(4-(aminomethyl)-1-oxo-1,2- dihydrophthalazin...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu... | Citation and Details BindingDB Entry DOI: 10.7270/Q2V128N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM515317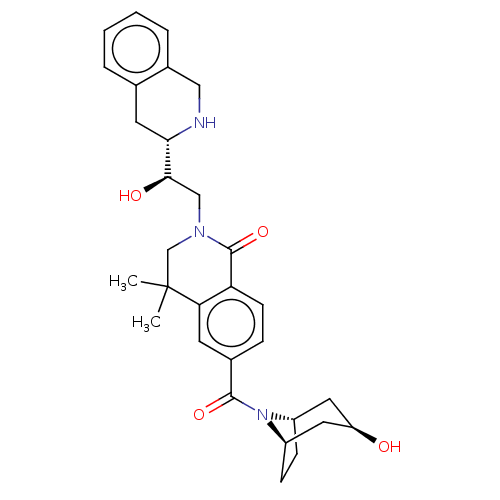 (US11098059, Example 21) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ... | Citation and Details BindingDB Entry DOI: 10.7270/Q26D5X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM515315 (US11098059, Example 19) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ... | Citation and Details BindingDB Entry DOI: 10.7270/Q26D5X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM578885 (4-(aminomethyl)-6-(5-(2,4- dimethylphenoxy)pyridin...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu... | Citation and Details BindingDB Entry DOI: 10.7270/Q2V128N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM578922 (4-(aminomethyl)-6-(3-(methoxymethyl)-1H- pyrrolo[2...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assay uses purified human, PRMT5 enzyme to convert S-adenosyl-L-[methyl-3H]methionine plus histone H4 L-arginine to S-adenosyl-L-homocysteine plu... | Citation and Details BindingDB Entry DOI: 10.7270/Q2V128N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50523663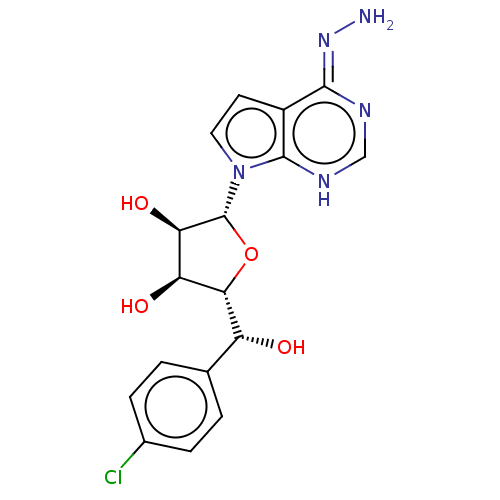 (CHEMBL4536042) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Prelude Therapeutics Curated by ChEMBL | Assay Description Inhibition of PRMT5 (unknown origin)/MEP50 (unknown origin) using histone H2 as substrate preincubated for 15 to 20 mins followed by S-[methyl-3H]ade... | Bioorg Med Chem Lett 29: 1264-1269 (2019) Article DOI: 10.1016/j.bmcl.2019.03.042 BindingDB Entry DOI: 10.7270/Q2ZC869N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3346 total ) | Next | Last >> |