

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urease subunit alpha [F36L]/beta/gamma (Proteus vulgaris) | BDBM24969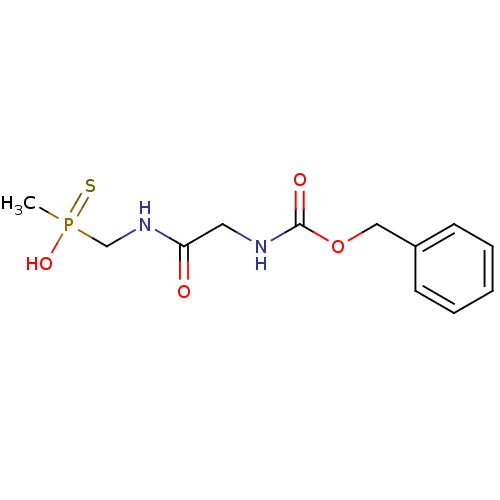 ([(2-{[(benzyloxy)carbonyl]amino}acetamido)methyl](...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 450 | -8.80 | 3.10E+3 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha [F36L]/beta/gamma (Proteus vulgaris) | BDBM24962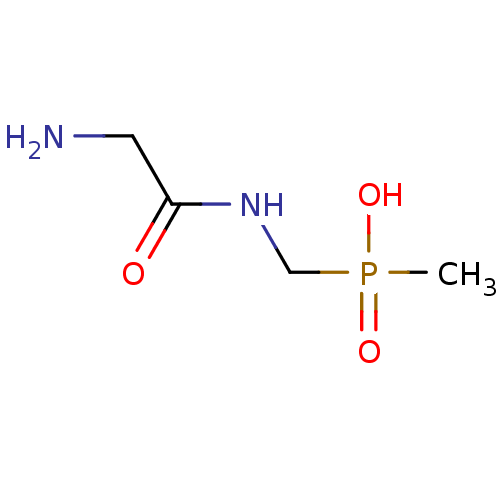 ([(2-aminoacetamido)methyl](methyl)phosphinic acid ...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.00E+4 | -6.27 | 8.60E+4 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha [F36L]/beta/gamma (Proteus vulgaris) | BDBM24967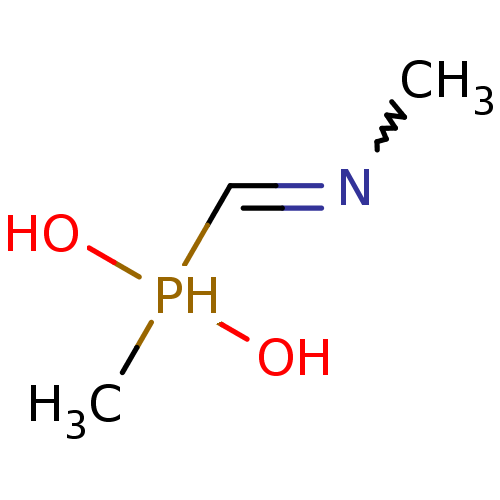 (methyl[(methylamino)methyl]phosphinic acid | organ...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.70E+4 | -6.33 | 1.53E+5 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha [F36L]/beta/gamma (Proteus vulgaris) | BDBM24971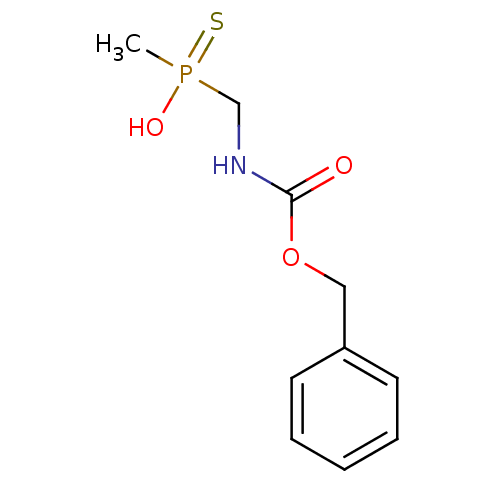 (({[(benzyloxy)carbonyl]amino}methyl)(methyl)sulfan...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40E+4 | -6.73 | 1.58E+5 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha [F36L]/beta/gamma (Proteus vulgaris) | BDBM24965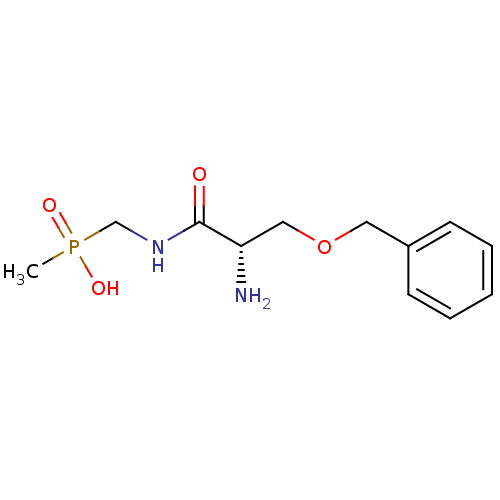 (organophosphorus derivative, 6 | {[(2S)-2-amino-3-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.26E+4 | -6.22 | 1.83E+5 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha [F36L]/beta/gamma (Proteus vulgaris) | BDBM24968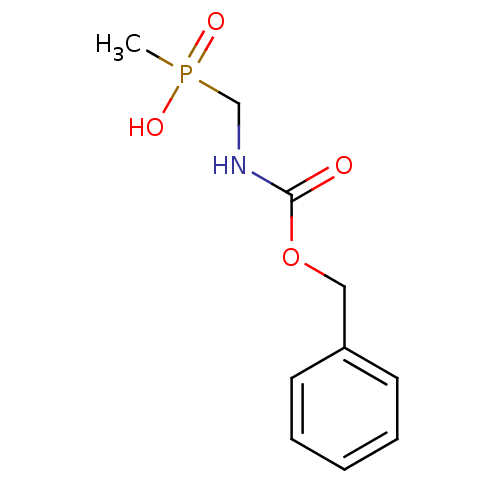 (({[(benzyloxy)carbonyl]amino}methyl)(methyl)phosph...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 6.50E+4 | -5.80 | 3.19E+5 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha [F36L]/beta/gamma (Proteus vulgaris) | BDBM24966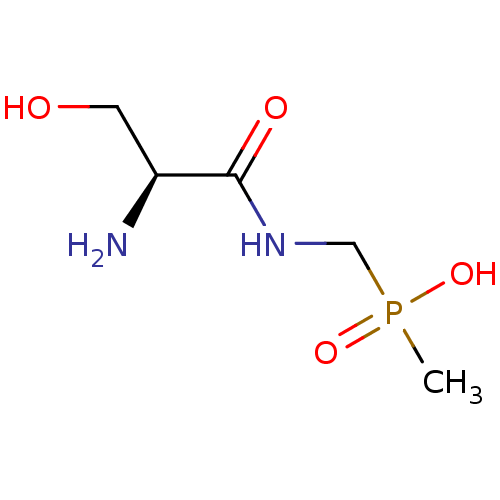 (organophosphorus derivative, 7 | {[(2S)-2-amino-3-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.10E+4 | -6.08 | 3.42E+5 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha [F36L]/beta/gamma (Proteus vulgaris) | BDBM24963 (organophosphorus derivative, 4 | {[(2S)-2-amino-3-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.08E+5 | -5.10 | 6.17E+5 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha [F36L]/beta/gamma (Proteus vulgaris) | BDBM24970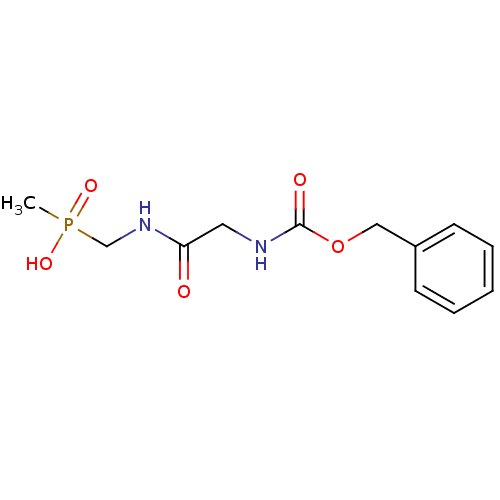 ([(2-{[(benzyloxy)carbonyl]amino}acetamido)methyl](...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.78E+5 | -5.20 | 6.40E+5 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha [F36L]/beta/gamma (Proteus vulgaris) | BDBM24964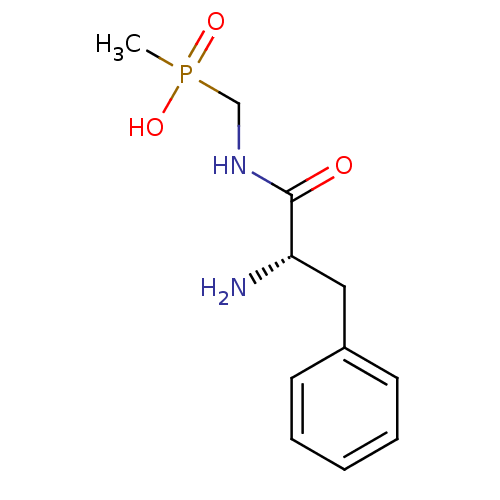 (organophosphorus derivative, 5 | {[(2S)-2-amino-3-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.76E+5 | -5.20 | 7.54E+5 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha [F36L]/beta/gamma (Proteus vulgaris) | BDBM24960 ((aminomethyl)(methyl)phosphinic acid | organophosp...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 4.25E+5 | -4.67 | 2.53E+6 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||