 Found 477 hits of ic50 for UniProtKB: P25095
Found 477 hits of ic50 for UniProtKB: P25095 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50030815
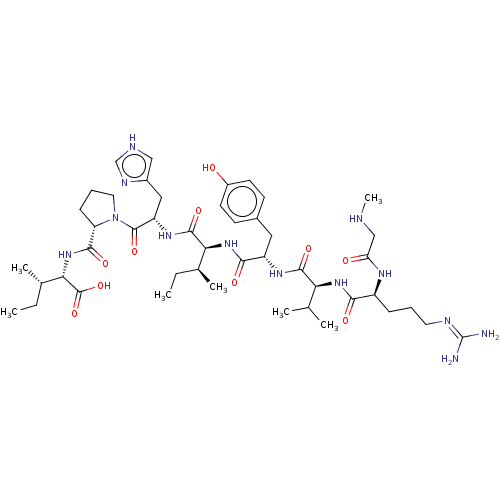
(CHEMBL404594)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)[C@@H](C)CC)C(O)=O |wU:8.7,25.27,wD:41.44,4.4,15.16,29.31,45.48,2.2,62.65,(8.44,-18.06,;9.36,-17.24,;9.05,-15.73,;7.88,-15.34,;10.19,-14.7,;9.88,-13.19,;11.03,-12.16,;12.2,-12.55,;10.71,-10.66,;11.74,-9.51,;10.97,-8.18,;9.46,-8.5,;9.33,-10.02,;7.99,-10.79,;7.99,-12.02,;6.66,-10.02,;5.32,-10.79,;5.32,-12.33,;4.07,-13.22,;4.55,-14.68,;6.09,-14.68,;6.56,-13.21,;6.66,-8.48,;5.33,-7.71,;4.43,-8.22,;5.33,-6.17,;3.99,-5.39,;4,-3.85,;5.06,-3.24,;2.66,-3.08,;1.33,-3.85,;-.01,-3.08,;-1.34,-3.84,;-2.67,-3.07,;-2.67,-1.53,;-3.73,-.91,;-1.33,-.76,;,-1.54,;2.67,-1.54,;4,-.77,;5.07,-1.39,;4.01,.77,;5.35,1.53,;5.36,3.07,;4.29,3.69,;6.69,3.84,;6.7,5.37,;8.04,6.14,;8.04,7.68,;9.38,8.44,;9.39,9.99,;10.45,10.6,;8.32,10.61,;8.03,3.06,;8.02,1.52,;6.94,.91,;9.35,.74,;9.34,-.8,;10.22,-1.32,;2.68,1.54,;1.61,.93,;2.69,2.78,;6.66,-5.4,;7.73,-6.01,;6.66,-3.86,;7.73,-3.24,;11.66,-15.17,;11.91,-16.38,;12.58,-14.35,)| Show InChI InChI=1S/C46H73N13O10/c1-8-26(5)37(43(66)55-33(21-29-22-50-24-52-29)44(67)59-19-11-13-34(59)41(64)58-38(45(68)69)27(6)9-2)57-40(63)32(20-28-14-16-30(60)17-15-28)54-42(65)36(25(3)4)56-39(62)31(53-35(61)23-49-7)12-10-18-51-46(47)48/h14-17,22,24-27,31-34,36-38,49,60H,8-13,18-21,23H2,1-7H3,(H,50,52)(H,53,61)(H,54,65)(H,55,66)(H,56,62)(H,57,63)(H,58,64)(H,68,69)(H4,47,48,51)/t26-,27-,31-,32-,33-,34-,36-,37-,38-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity for rat brain Angiotensin II receptor |
J Med Chem 34: 3248-60 (1991)
BindingDB Entry DOI: 10.7270/Q24X5B06 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230790

(CHEMBL292892)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1[N+]([O-])=O)C(O)=O Show InChI InChI=1S/C23H23N3O6S/c1-2-3-6-21-24-13-18(10-17(23(29)30)11-19-5-4-9-33-19)25(21)14-16-8-7-15(22(27)28)12-20(16)26(31)32/h4-5,7-10,12-13H,2-3,6,11,14H2,1H3,(H,27,28)(H,29,30)/b17-10+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230779

(CHEMBL294686)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)c2nn[nH]n2)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24N6O2S/c1-2-3-6-21-24-14-19(29(21)15-16-7-9-17(10-8-16)23(30)31)12-18(22-25-27-28-26-22)13-20-5-4-11-32-20/h4-5,7-12,14H,2-3,6,13,15H2,1H3,(H,30,31)(H,25,26,27,28)/b18-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50030815

(CHEMBL404594)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)[C@@H](C)CC)C(O)=O |wU:8.7,25.27,wD:41.44,4.4,15.16,29.31,45.48,2.2,62.65,(8.44,-18.06,;9.36,-17.24,;9.05,-15.73,;7.88,-15.34,;10.19,-14.7,;9.88,-13.19,;11.03,-12.16,;12.2,-12.55,;10.71,-10.66,;11.74,-9.51,;10.97,-8.18,;9.46,-8.5,;9.33,-10.02,;7.99,-10.79,;7.99,-12.02,;6.66,-10.02,;5.32,-10.79,;5.32,-12.33,;4.07,-13.22,;4.55,-14.68,;6.09,-14.68,;6.56,-13.21,;6.66,-8.48,;5.33,-7.71,;4.43,-8.22,;5.33,-6.17,;3.99,-5.39,;4,-3.85,;5.06,-3.24,;2.66,-3.08,;1.33,-3.85,;-.01,-3.08,;-1.34,-3.84,;-2.67,-3.07,;-2.67,-1.53,;-3.73,-.91,;-1.33,-.76,;,-1.54,;2.67,-1.54,;4,-.77,;5.07,-1.39,;4.01,.77,;5.35,1.53,;5.36,3.07,;4.29,3.69,;6.69,3.84,;6.7,5.37,;8.04,6.14,;8.04,7.68,;9.38,8.44,;9.39,9.99,;10.45,10.6,;8.32,10.61,;8.03,3.06,;8.02,1.52,;6.94,.91,;9.35,.74,;9.34,-.8,;10.22,-1.32,;2.68,1.54,;1.61,.93,;2.69,2.78,;6.66,-5.4,;7.73,-6.01,;6.66,-3.86,;7.73,-3.24,;11.66,-15.17,;11.91,-16.38,;12.58,-14.35,)| Show InChI InChI=1S/C46H73N13O10/c1-8-26(5)37(43(66)55-33(21-29-22-50-24-52-29)44(67)59-19-11-13-34(59)41(64)58-38(45(68)69)27(6)9-2)57-40(63)32(20-28-14-16-30(60)17-15-28)54-42(65)36(25(3)4)56-39(62)31(53-35(61)23-49-7)12-10-18-51-46(47)48/h14-17,22,24-27,31-34,36-38,49,60H,8-13,18-21,23H2,1-7H3,(H,50,52)(H,53,61)(H,54,65)(H,55,66)(H,56,62)(H,57,63)(H,58,64)(H,68,69)(H4,47,48,51)/t26-,27-,31-,32-,33-,34-,36-,37-,38-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity against Angiotensin II receptor, from rat adrenal gland |
J Med Chem 34: 3248-60 (1991)
BindingDB Entry DOI: 10.7270/Q24X5B06 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230811
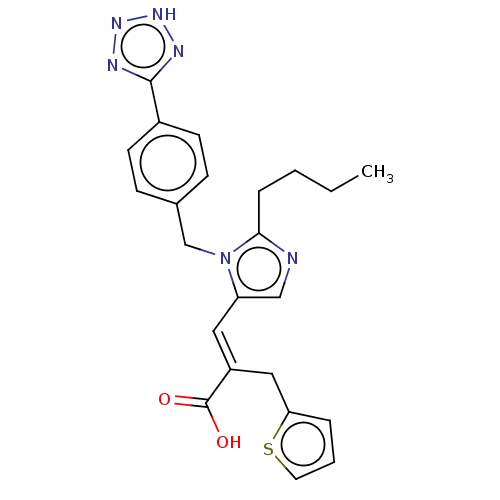
(CHEMBL293091)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)-c1nn[nH]n1 Show InChI InChI=1S/C23H24N6O2S/c1-2-3-6-21-24-14-19(12-18(23(30)31)13-20-5-4-11-32-20)29(21)15-16-7-9-17(10-8-16)22-25-27-28-26-22/h4-5,7-12,14H,2-3,6,13,15H2,1H3,(H,30,31)(H,25,26,27,28)/b18-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230778
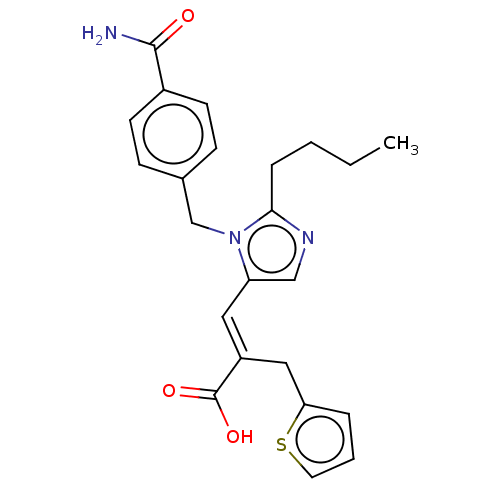
(CHEMBL56211)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)C(N)=O Show InChI InChI=1S/C23H25N3O3S/c1-2-3-6-21-25-14-19(12-18(23(28)29)13-20-5-4-11-30-20)26(21)15-16-7-9-17(10-8-16)22(24)27/h4-5,7-12,14H,2-3,6,13,15H2,1H3,(H2,24,27)(H,28,29)/b18-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230771
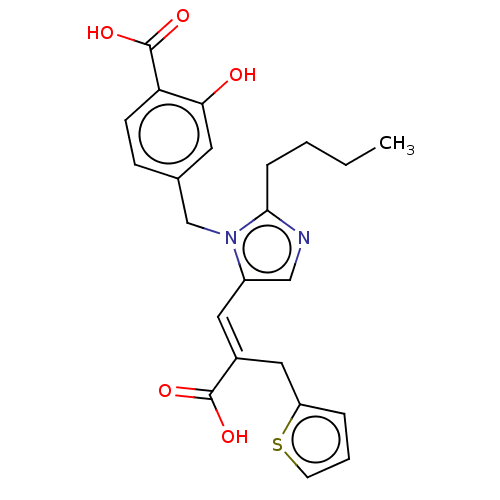
(CHEMBL55510)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(C(O)=O)c(O)c1 Show InChI InChI=1S/C23H24N2O5S/c1-2-3-6-21-24-13-17(11-16(22(27)28)12-18-5-4-9-31-18)25(21)14-15-7-8-19(23(29)30)20(26)10-15/h4-5,7-11,13,26H,2-3,6,12,14H2,1H3,(H,27,28)(H,29,30)/b16-11+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230806

(CHEMBL294415)Show SMILES CCCCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C25H28N2O4S/c1-2-3-4-5-8-23-26-16-21(14-20(25(30)31)15-22-7-6-13-32-22)27(23)17-18-9-11-19(12-10-18)24(28)29/h6-7,9-14,16H,2-5,8,15,17H2,1H3,(H,28,29)(H,30,31)/b20-14+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50048078

(4-[2-Butyl-5-((E)-2-carboxy-3-thiophen-2-yl-propen...)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(C(O)=O)c(Cl)c1 Show InChI InChI=1S/C23H23ClN2O4S/c1-2-3-6-21-25-13-17(11-16(22(27)28)12-18-5-4-9-31-18)26(21)14-15-7-8-19(23(29)30)20(24)10-15/h4-5,7-11,13H,2-3,6,12,14H2,1H3,(H,27,28)(H,29,30)/b16-11+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50212829
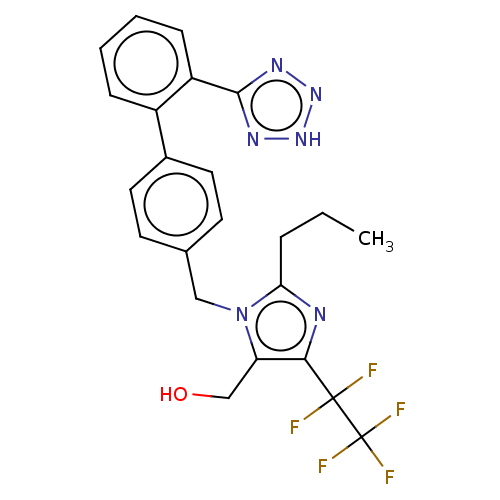
(CHEMBL170944)Show SMILES CCCc1nc(c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)C(F)(F)C(F)(F)F Show InChI InChI=1S/C23H21F5N6O/c1-2-5-19-29-20(22(24,25)23(26,27)28)18(13-35)34(19)12-14-8-10-15(11-9-14)16-6-3-4-7-17(16)21-30-32-33-31-21/h3-4,6-11,35H,2,5,12-13H2,1H3,(H,30,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Angiotensin II receptor antagonist activity was determined by 50% inhibition of specific binding of [3H]angiotensin II (2 nM) to rat adrenal cortical... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2T72KMB |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50042235

(2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...)Show SMILES CCCCC1=NC2(CCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4| Show InChI InChI=1S/C25H28N6O/c1-2-3-10-22-26-25(15-6-7-16-25)24(32)31(22)17-18-11-13-19(14-12-18)20-8-4-5-9-21(20)23-27-29-30-28-23/h4-5,8-9,11-14H,2-3,6-7,10,15-17H2,1H3,(H,27,28,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of [125I]-AII binding to Angiotensin II receptor of rat uterine membrane |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2JQ135P |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50228195

(Angiotensin Ii | CHEBI:2719)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C50H71N13O12/c1-5-28(4)41(47(72)59-36(23-31-25-54-26-56-31)48(73)63-20-10-14-38(63)45(70)60-37(49(74)75)22-29-11-7-6-8-12-29)62-44(69)35(21-30-15-17-32(64)18-16-30)58-46(71)40(27(2)3)61-43(68)34(13-9-19-55-50(52)53)57-42(67)33(51)24-39(65)66/h6-8,11-12,15-18,25-28,33-38,40-41,64H,5,9-10,13-14,19-24,51H2,1-4H3,(H,54,56)(H,57,67)(H,58,71)(H,59,72)(H,60,70)(H,61,68)(H,62,69)(H,65,66)(H,74,75)(H4,52,53,55)/t28-,33-,34-,35-,36-,37-,38-,40-,41-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity against Angiotensin II receptor, from rat adrenal gland |
J Med Chem 34: 3248-60 (1991)
BindingDB Entry DOI: 10.7270/Q24X5B06 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50011975

(4-[2-Butyl-5-((E)-2-carboxy-3-thiophen-2-yl-propen...)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1Cl)C(O)=O Show InChI InChI=1S/C23H23ClN2O4S/c1-2-3-6-21-25-13-18(10-17(23(29)30)11-19-5-4-9-31-19)26(21)14-16-8-7-15(22(27)28)12-20(16)24/h4-5,7-10,12-13H,2-3,6,11,14H2,1H3,(H,27,28)(H,29,30)/b17-10+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50228894

(CHEMBL312754)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O |wU:57.60,4.4,44.44,24.25,wD:20.21,8.8,61.64,2.2,(10.41,1.37,;9.34,.76,;9.34,-.78,;10.41,-1.4,;8.01,-1.55,;6.67,-.78,;5.34,-1.55,;5.33,-2.78,;4,-.77,;2.67,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;-1.34,.77,;-2.41,1.39,;-1.34,-.77,;,-1.54,;4.01,.77,;5.35,1.53,;6.41,.91,;5.35,3.07,;6.69,3.84,;8.02,3.06,;8.02,1.83,;9.36,3.83,;10.7,3.06,;12.03,3.82,;13.37,3.05,;14.71,3.81,;16.04,3.04,;17.11,3.65,;16.03,1.81,;9.36,5.37,;8.03,6.14,;7.14,5.63,;8.03,7.68,;6.7,8.46,;6.7,9.48,;4.02,3.85,;2.95,3.24,;4.03,5.08,;8,-3.09,;6.94,-3.71,;9.34,-3.87,;9.33,-5.41,;10.67,-6.18,;12,-5.42,;13.4,-6.06,;14.43,-4.91,;13.66,-3.58,;12.15,-3.89,;8,-6.18,;7.11,-5.67,;8,-7.72,;9.25,-8.61,;8.77,-10.07,;7.23,-10.07,;6.76,-8.61,;5.3,-8.12,;5.05,-6.92,;4.14,-9.14,;2.68,-8.65,;2.44,-7.45,;1.52,-9.68,;1.73,-10.68,;.36,-9.29,)| Show InChI InChI=1S/C43H67N13O10/c1-7-24(4)35(40(63)53-31(19-27-20-47-22-49-27)41(64)56-17-9-11-32(56)38(61)50-25(5)42(65)66)55-37(60)30(18-26-12-14-28(57)15-13-26)52-39(62)34(23(2)3)54-36(59)29(51-33(58)21-46-6)10-8-16-48-43(44)45/h12-15,20,22-25,29-32,34-35,46,57H,7-11,16-19,21H2,1-6H3,(H,47,49)(H,50,61)(H,51,58)(H,52,62)(H,53,63)(H,54,59)(H,55,60)(H,65,66)(H4,44,45,48)/t24-,25-,29-,30-,31-,32-,34-,35-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity against Angiotensin II receptor, from rat adrenal gland |
J Med Chem 34: 3248-60 (1991)
BindingDB Entry DOI: 10.7270/Q24X5B06 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50236697

(5-L-isoleucineangiotensin II | 5-isoleucine-angiot...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |wU:4.4,47.47,60.63,35.36,20.21,wD:2.2,24.32,8.17,64.66,(24.13,1.24,;24.1,-.31,;25.42,-1.09,;26.77,-.35,;25.4,-2.63,;24.05,-3.38,;22.73,-2.59,;22.75,-1.05,;21.38,-3.34,;21.36,-4.88,;22.68,-5.66,;24.01,-4.93,;25.33,-5.71,;25.32,-7.24,;26.64,-8.05,;23.97,-8.01,;22.65,-7.22,;20.06,-2.55,;18.71,-3.29,;18.68,-4.83,;17.38,-2.5,;16.04,-3.25,;14.72,-2.46,;14.74,-.92,;13.37,-3.2,;13.34,-4.74,;14.66,-5.53,;14.64,-7.08,;15.96,-7.87,;15.93,-9.4,;14.59,-10.16,;17.25,-10.21,;12.05,-2.42,;10.69,-3.15,;10.67,-4.69,;9.37,-2.36,;8.03,-3.1,;9.4,-.82,;10.75,-.07,;12.07,-.88,;10.78,1.47,;17.41,-.96,;18.76,-.21,;16.1,-.17,;26.72,-3.43,;26.69,-4.97,;28.07,-2.68,;29.38,-3.48,;30.73,-2.73,;30.75,-1.19,;29.53,-.27,;30.03,1.18,;31.57,1.17,;32.02,-.31,;29.35,-5.02,;28.01,-5.76,;30.54,-5.79,;31.99,-5.25,;32.95,-6.46,;32.1,-7.74,;30.62,-7.33,;29.33,-8.36,;27.89,-7.82,;29.58,-9.88,;28.39,-10.86,;26.95,-10.32,;25.76,-11.3,;26.02,-12.81,;24.84,-13.79,;23.4,-13.26,;23.14,-11.74,;24.32,-10.75,;28.64,-12.38,;27.45,-13.36,;30.08,-12.92,)| Show InChI InChI=1S/C50H71N13O12/c1-5-28(4)41(47(72)59-36(23-31-25-54-26-56-31)48(73)63-20-10-14-38(63)45(70)60-37(49(74)75)22-29-11-7-6-8-12-29)62-44(69)35(21-30-15-17-32(64)18-16-30)58-46(71)40(27(2)3)61-43(68)34(13-9-19-55-50(52)53)57-42(67)33(51)24-39(65)66/h6-8,11-12,15-18,25-28,33-38,40-41,64H,5,9-10,13-14,19-24,51H2,1-4H3,(H,54,56)(H,57,67)(H,58,71)(H,59,72)(H,60,70)(H,61,68)(H,62,69)(H,65,66)(H,74,75)(H4,52,53,55)/t28-,33-,34-,35-,36-,37-,38-,40-,41-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Angiotensin II receptor antagonist activity was determined by 50% inhibition of specific binding of [3H]angiotensin II (2 nM) to rat adrenal cortical... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2T72KMB |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50281848
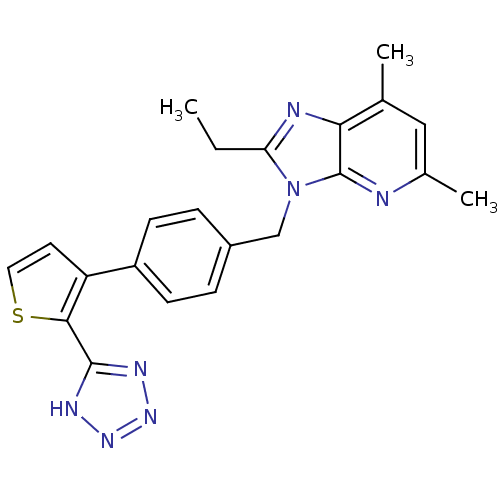
(2-Ethyl-5,7-dimethyl-3-{4-[2-(2H-tetrazol-5-yl)-5H...)Show SMILES CCc1nc2c(C)cc(C)nc2n1Cc1ccc(cc1)-c1ccsc1-c1nnn[nH]1 Show InChI InChI=1S/C22H21N7S/c1-4-18-24-19-13(2)11-14(3)23-22(19)29(18)12-15-5-7-16(8-6-15)17-9-10-30-20(17)21-25-27-28-26-21/h5-11H,4,12H2,1-3H3,(H,25,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compound was tested for inhibition of Angiotensin II receptor in rabbit aorta binding assay |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2DZ0BFZ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50212853

(CHEMBL274626)Show SMILES CCc1nc2c(C)cc(C)nc2n1Cc1ccc(cc1)-c1cscc1-c1nn[nH]n1 Show InChI InChI=1S/C22H21N7S/c1-4-19-24-20-13(2)9-14(3)23-22(20)29(19)10-15-5-7-16(8-6-15)17-11-30-12-18(17)21-25-27-28-26-21/h5-9,11-12H,4,10H2,1-3H3,(H,25,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compound was tested for inhibition of Angiotensin II receptor in rabbit aorta binding assay |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2DZ0BFZ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230787

(CHEMBL54421)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(C(O)=O)c(c1)-c1ccccc1 Show InChI InChI=1S/C29H28N2O4S/c1-2-3-11-27-30-18-23(16-22(28(32)33)17-24-10-7-14-36-24)31(27)19-20-12-13-25(29(34)35)26(15-20)21-8-5-4-6-9-21/h4-10,12-16,18H,2-3,11,17,19H2,1H3,(H,32,33)(H,34,35)/b22-16+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50212825
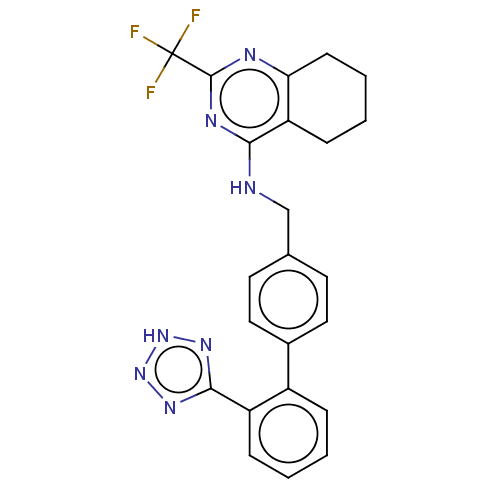
(CHEMBL435855)Show SMILES FC(F)(F)c1nc2CCCCc2c(NCc2ccc(cc2)-c2ccccc2-c2nn[nH]n2)n1 Show InChI InChI=1S/C23H20F3N7/c24-23(25,26)22-28-19-8-4-3-7-18(19)20(29-22)27-13-14-9-11-15(12-10-14)16-5-1-2-6-17(16)21-30-32-33-31-21/h1-2,5-6,9-12H,3-4,7-8,13H2,(H,27,28,29)(H,30,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of Angiotensin II receptor binding of radiolabeled AII to rat adrenal glomerulosa tissue by 50% |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z03B9M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230792
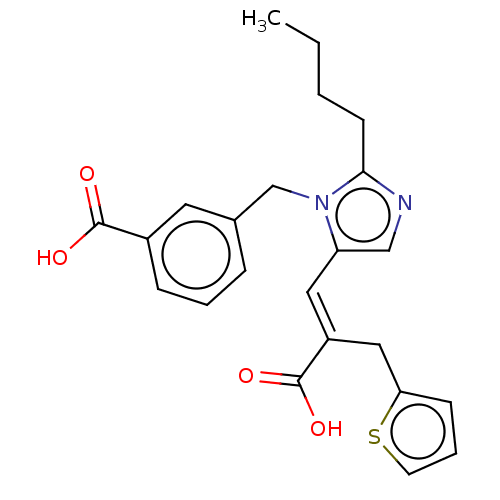
(CHEMBL56497)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1cccc(c1)C(O)=O Show InChI InChI=1S/C23H24N2O4S/c1-2-3-9-21-24-14-19(12-18(23(28)29)13-20-8-5-10-30-20)25(21)15-16-6-4-7-17(11-16)22(26)27/h4-8,10-12,14H,2-3,9,13,15H2,1H3,(H,26,27)(H,28,29)/b18-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230799
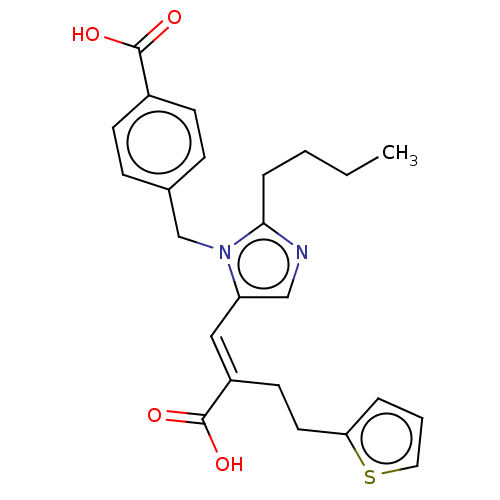
(CHEMBL56333)Show SMILES CCCCc1ncc(\C=C(/CCc2cccs2)C(O)=O)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C24H26N2O4S/c1-2-3-6-22-25-15-20(14-19(24(29)30)11-12-21-5-4-13-31-21)26(22)16-17-7-9-18(10-8-17)23(27)28/h4-5,7-10,13-15H,2-3,6,11-12,16H2,1H3,(H,27,28)(H,29,30)/b19-14+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50228195

(Angiotensin Ii | CHEBI:2719)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C50H71N13O12/c1-5-28(4)41(47(72)59-36(23-31-25-54-26-56-31)48(73)63-20-10-14-38(63)45(70)60-37(49(74)75)22-29-11-7-6-8-12-29)62-44(69)35(21-30-15-17-32(64)18-16-30)58-46(71)40(27(2)3)61-43(68)34(13-9-19-55-50(52)53)57-42(67)33(51)24-39(65)66/h6-8,11-12,15-18,25-28,33-38,40-41,64H,5,9-10,13-14,19-24,51H2,1-4H3,(H,54,56)(H,57,67)(H,58,71)(H,59,72)(H,60,70)(H,61,68)(H,62,69)(H,65,66)(H,74,75)(H4,52,53,55)/t28-,33-,34-,35-,36-,37-,38-,40-,41-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity for rat brain Angiotensin II receptor |
J Med Chem 34: 3248-60 (1991)
BindingDB Entry DOI: 10.7270/Q24X5B06 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230774
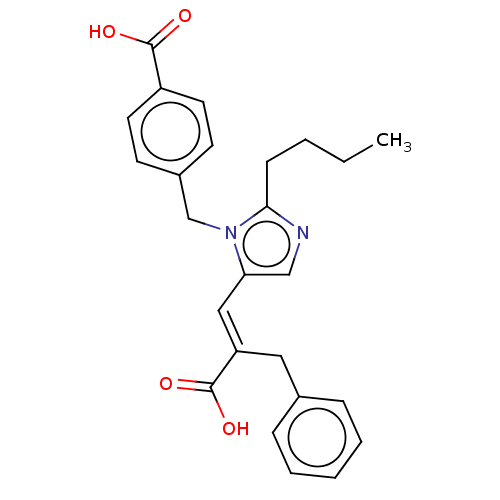
(CHEMBL291955)Show SMILES CCCCc1ncc(\C=C(/Cc2ccccc2)C(O)=O)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C25H26N2O4/c1-2-3-9-23-26-16-22(15-21(25(30)31)14-18-7-5-4-6-8-18)27(23)17-19-10-12-20(13-11-19)24(28)29/h4-8,10-13,15-16H,2-3,9,14,17H2,1H3,(H,28,29)(H,30,31)/b21-15+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230762

(CHEMBL56160)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C22H25N3O4S2/c1-2-3-6-21-24-14-18(12-17(22(26)27)13-19-5-4-11-30-19)25(21)15-16-7-9-20(10-8-16)31(23,28)29/h4-5,7-12,14H,2-3,6,13,15H2,1H3,(H,26,27)(H2,23,28,29)/b17-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50212843
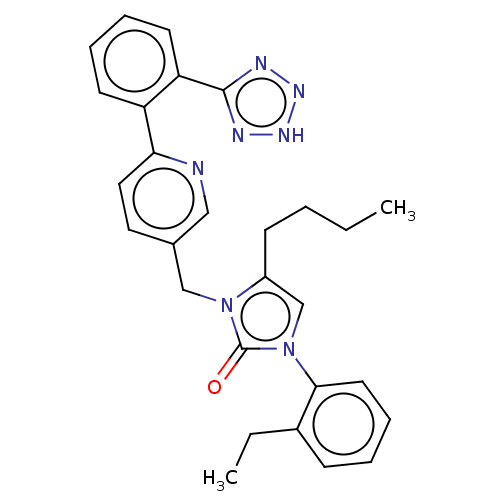
(CHEMBL267809)Show SMILES CCCCc1cn(-c2ccccc2CC)c(=O)n1Cc1ccc(nc1)-c1ccccc1-c1nn[nH]n1 Show InChI InChI=1S/C28H29N7O/c1-3-5-11-22-19-35(26-14-9-6-10-21(26)4-2)28(36)34(22)18-20-15-16-25(29-17-20)23-12-7-8-13-24(23)27-30-32-33-31-27/h6-10,12-17,19H,3-5,11,18H2,1-2H3,(H,30,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of [125I]-AII binding to Angiotensin II receptor of rat uterine membrane |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2JQ135P |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230810
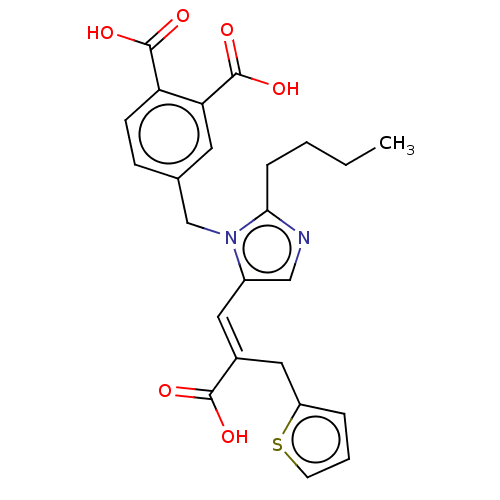
(CHEMBL299064)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(C(O)=O)c(c1)C(O)=O Show InChI InChI=1S/C24H24N2O6S/c1-2-3-6-21-25-13-17(11-16(22(27)28)12-18-5-4-9-33-18)26(21)14-15-7-8-19(23(29)30)20(10-15)24(31)32/h4-5,7-11,13H,2-3,6,12,14H2,1H3,(H,27,28)(H,29,30)(H,31,32)/b16-11+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM82258

(CAS_114798-26-4 | Losartan | NSC_3961)Show SMILES CCCCc1nc(Cl)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H23ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3,(H,25,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AII from the Angiotensin II receptor isolated from the liver of rats |
J Med Chem 38: 4670-8 (1995)
Article DOI: 10.1021/jm00023a006
BindingDB Entry DOI: 10.7270/Q21J9DHJ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230764
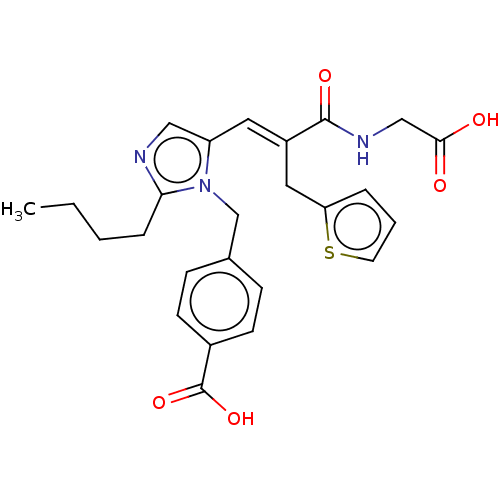
(CHEMBL299954)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(=O)NCC(O)=O)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C25H27N3O5S/c1-2-3-6-22-26-14-20(28(22)16-17-7-9-18(10-8-17)25(32)33)12-19(13-21-5-4-11-34-21)24(31)27-15-23(29)30/h4-5,7-12,14H,2-3,6,13,15-16H2,1H3,(H,27,31)(H,29,30)(H,32,33)/b19-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50281823
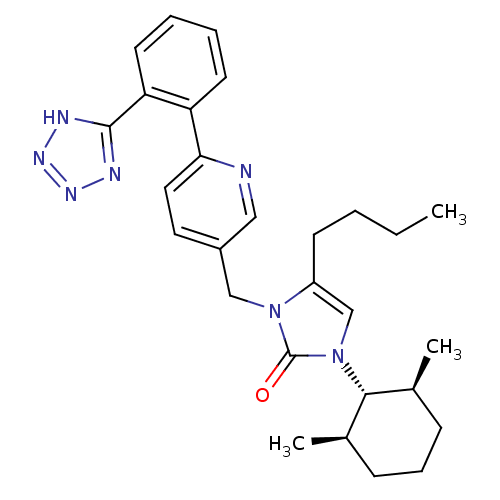
(4-Butyl-1-((1R,2R,6S)-2,6-dimethyl-cyclohexyl)-3-{...)Show SMILES CCCCc1cn([C@@H]2[C@@H](C)CCC[C@H]2C)c(=O)n1Cc1ccc(nc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C28H35N7O/c1-4-5-11-22-18-35(26-19(2)9-8-10-20(26)3)28(36)34(22)17-21-14-15-25(29-16-21)23-12-6-7-13-24(23)27-30-32-33-31-27/h6-7,12-16,18-20,26H,4-5,8-11,17H2,1-3H3,(H,30,31,32,33)/t19-,20+,26+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of [125I]-AII binding to Angiotensin II receptor of rat uterine membrane |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2JQ135P |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50212845

(CHEMBL274668)Show SMILES CCCCc1cn(-c2c(CC)cccc2CC)c(=O)n1Cc1ccc(nc1)-c1ccccc1-c1nn[nH]n1 |(.63,-7.73,;-.9,-7.51,;-1.46,-6.08,;-3,-5.83,;-3.56,-4.39,;-5.03,-3.99,;-5.12,-2.47,;-6.45,-1.7,;-6.45,-.15,;-5.12,.62,;-5.14,2.16,;-7.79,.62,;-9.12,-.16,;-9.12,-1.7,;-7.79,-2.47,;-7.79,-4.01,;-9.12,-4.77,;-3.7,-1.91,;-3.3,-.4,;-2.72,-3.08,;-1.18,-3,;-.48,-1.64,;1.06,-1.55,;1.75,-.17,;.92,1.13,;-.62,1.04,;-1.32,-.33,;1.61,2.49,;3.15,2.58,;3.85,3.95,;3.01,5.26,;1.48,5.16,;.78,3.79,;-.76,3.7,;-1.54,2.39,;-3.04,2.74,;-3.18,4.24,;-1.79,4.86,)| Show InChI InChI=1S/C30H33N7O/c1-4-7-13-24-20-37(28-22(5-2)11-10-12-23(28)6-3)30(38)36(24)19-21-16-17-27(31-18-21)25-14-8-9-15-26(25)29-32-34-35-33-29/h8-12,14-18,20H,4-7,13,19H2,1-3H3,(H,32,33,34,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of [125I]-AII binding to Angiotensin II receptor of rat uterine membrane |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2JQ135P |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50212849
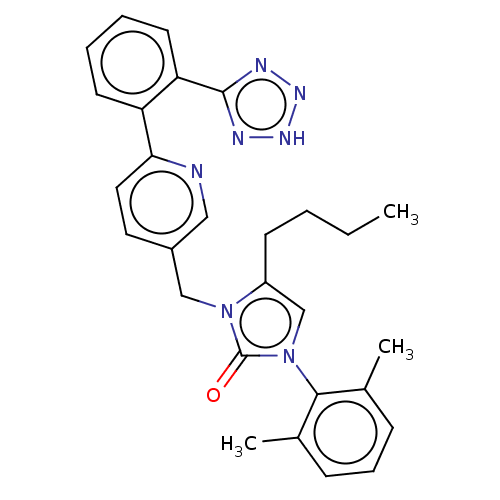
(CHEMBL430152)Show SMILES CCCCc1cn(-c2c(C)cccc2C)c(=O)n1Cc1ccc(nc1)-c1ccccc1-c1nn[nH]n1 |(.63,-7.72,;-.9,-7.5,;-1.46,-6.07,;-2.99,-5.82,;-3.55,-4.38,;-5.03,-3.99,;-5.12,-2.47,;-6.44,-1.7,;-7.79,-2.47,;-7.79,-4,;-9.11,-1.7,;-9.11,-.16,;-7.79,.62,;-6.44,-.15,;-5.12,.62,;-3.69,-1.91,;-3.3,-.4,;-2.71,-3.08,;-1.18,-3,;-.48,-1.64,;1.05,-1.54,;1.75,-.17,;.92,1.13,;-.62,1.04,;-1.32,-.33,;1.61,2.49,;3.15,2.57,;3.85,3.95,;3.01,5.25,;1.47,5.16,;.78,3.78,;-.76,3.7,;-1.54,2.39,;-3.04,2.74,;-3.18,4.23,;-1.78,4.85,)| Show InChI InChI=1S/C28H29N7O/c1-4-5-11-22-18-35(26-19(2)9-8-10-20(26)3)28(36)34(22)17-21-14-15-25(29-16-21)23-12-6-7-13-24(23)27-30-32-33-31-27/h6-10,12-16,18H,4-5,11,17H2,1-3H3,(H,30,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of [125I]-AII binding to Angiotensin II receptor of rat uterine membrane |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2JQ135P |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230767
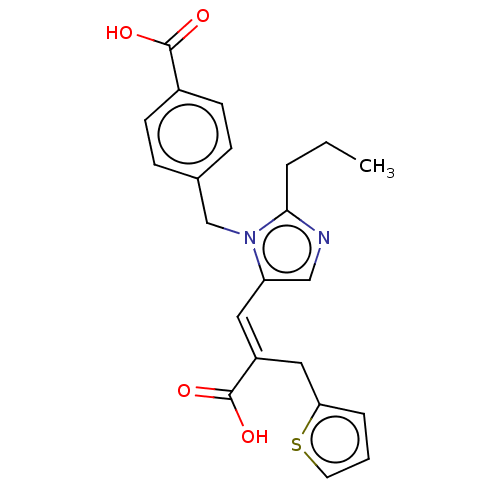
(CHEMBL56522)Show SMILES CCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C22H22N2O4S/c1-2-4-20-23-13-18(11-17(22(27)28)12-19-5-3-10-29-19)24(20)14-15-6-8-16(9-7-15)21(25)26/h3,5-11,13H,2,4,12,14H2,1H3,(H,25,26)(H,27,28)/b17-11+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50212952

(CHEMBL46150)Show SMILES CCCCc1nc2ccc(cc2c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)C1OCC=C1 |c:41| Show InChI InChI=1S/C30H28N6O2/c1-2-3-10-28-31-26-16-15-22(27-9-6-17-38-27)18-25(26)30(37)36(28)19-20-11-13-21(14-12-20)23-7-4-5-8-24(23)29-32-34-35-33-29/h4-9,11-16,18,27H,2-3,10,17,19H2,1H3,(H,32,33,34,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro Angiotensin II receptor antagonist activity in rat adrenal cortex tissue. |
Bioorg Med Chem Lett 4: 1703-1708 (1994)
Article DOI: 10.1016/S0960-894X(00)80365-0
BindingDB Entry DOI: 10.7270/Q2JS9QB3 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50228894

(CHEMBL312754)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O |wU:57.60,4.4,44.44,24.25,wD:20.21,8.8,61.64,2.2,(10.41,1.37,;9.34,.76,;9.34,-.78,;10.41,-1.4,;8.01,-1.55,;6.67,-.78,;5.34,-1.55,;5.33,-2.78,;4,-.77,;2.67,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;-1.34,.77,;-2.41,1.39,;-1.34,-.77,;,-1.54,;4.01,.77,;5.35,1.53,;6.41,.91,;5.35,3.07,;6.69,3.84,;8.02,3.06,;8.02,1.83,;9.36,3.83,;10.7,3.06,;12.03,3.82,;13.37,3.05,;14.71,3.81,;16.04,3.04,;17.11,3.65,;16.03,1.81,;9.36,5.37,;8.03,6.14,;7.14,5.63,;8.03,7.68,;6.7,8.46,;6.7,9.48,;4.02,3.85,;2.95,3.24,;4.03,5.08,;8,-3.09,;6.94,-3.71,;9.34,-3.87,;9.33,-5.41,;10.67,-6.18,;12,-5.42,;13.4,-6.06,;14.43,-4.91,;13.66,-3.58,;12.15,-3.89,;8,-6.18,;7.11,-5.67,;8,-7.72,;9.25,-8.61,;8.77,-10.07,;7.23,-10.07,;6.76,-8.61,;5.3,-8.12,;5.05,-6.92,;4.14,-9.14,;2.68,-8.65,;2.44,-7.45,;1.52,-9.68,;1.73,-10.68,;.36,-9.29,)| Show InChI InChI=1S/C43H67N13O10/c1-7-24(4)35(40(63)53-31(19-27-20-47-22-49-27)41(64)56-17-9-11-32(56)38(61)50-25(5)42(65)66)55-37(60)30(18-26-12-14-28(57)15-13-26)52-39(62)34(23(2)3)54-36(59)29(51-33(58)21-46-6)10-8-16-48-43(44)45/h12-15,20,22-25,29-32,34-35,46,57H,7-11,16-19,21H2,1-6H3,(H,47,49)(H,50,61)(H,51,58)(H,52,62)(H,53,63)(H,54,59)(H,55,60)(H,65,66)(H4,44,45,48)/t24-,25-,29-,30-,31-,32-,34-,35-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner Lambert Company
Curated by ChEMBL
| Assay Description
Binding affinity for rat brain Angiotensin II receptor |
J Med Chem 34: 3248-60 (1991)
BindingDB Entry DOI: 10.7270/Q24X5B06 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50046078
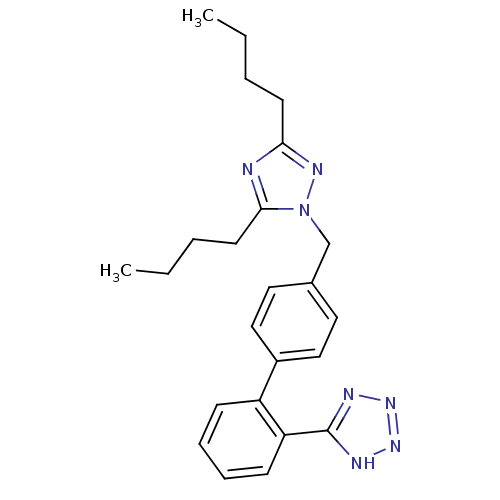
(5-[4'-(3,5-Dibutyl-[1,2,4]triazol-1-ylmethyl)-biph...)Show SMILES CCCCc1nc(CCCC)n(Cc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)n1 Show InChI InChI=1S/C24H29N7/c1-3-5-11-22-25-23(12-6-4-2)31(28-22)17-18-13-15-19(16-14-18)20-9-7-8-10-21(20)24-26-29-30-27-24/h7-10,13-16H,3-6,11-12,17H2,1-2H3,(H,26,27,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Tested in vitro for inhibition of [125I]AII to rat uterine membranes |
Citation and Details
Article DOI: 10.1016/S0960-894X(01)81129-X
BindingDB Entry DOI: 10.7270/Q2PN98BK |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50046078

(5-[4'-(3,5-Dibutyl-[1,2,4]triazol-1-ylmethyl)-biph...)Show SMILES CCCCc1nc(CCCC)n(Cc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)n1 Show InChI InChI=1S/C24H29N7/c1-3-5-11-22-25-23(12-6-4-2)31(28-22)17-18-13-15-19(16-14-18)20-9-7-8-10-21(20)24-26-29-30-27-24/h7-10,13-16H,3-6,11-12,17H2,1-2H3,(H,26,27,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of [125I]-AII binding to Angiotensin II receptor of rat uterine membrane |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2JQ135P |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50049207

(2-Butyl-6-(1-methoxy-1-methyl-ethyl)-3-[2'-(1H-tet...)Show SMILES CCCCc1nc2ccc(cc2c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)C(C)(C)OC Show InChI InChI=1S/C30H32N6O2/c1-5-6-11-27-31-26-17-16-22(30(2,3)38-4)18-25(26)29(37)36(27)19-20-12-14-21(15-13-20)23-9-7-8-10-24(23)28-32-34-35-33-28/h7-10,12-18H,5-6,11,19H2,1-4H3,(H,32,33,34,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro Angiotensin II receptor antagonist activity in rat adrenal cortex tissue. |
Bioorg Med Chem Lett 4: 1703-1708 (1994)
Article DOI: 10.1016/S0960-894X(00)80365-0
BindingDB Entry DOI: 10.7270/Q2JS9QB3 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50212830
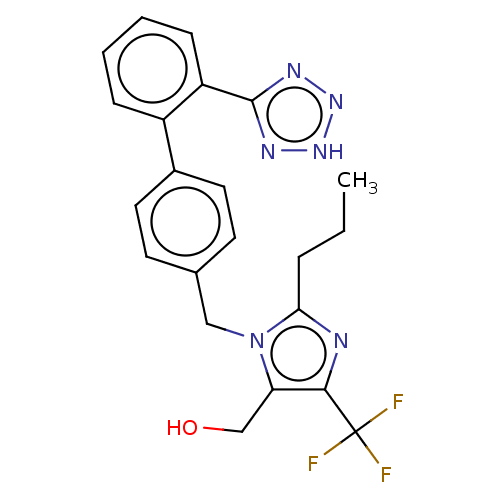
(CHEMBL171524)Show SMILES CCCc1nc(c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)C(F)(F)F Show InChI InChI=1S/C22H21F3N6O/c1-2-5-19-26-20(22(23,24)25)18(13-32)31(19)12-14-8-10-15(11-9-14)16-6-3-4-7-17(16)21-27-29-30-28-21/h3-4,6-11,32H,2,5,12-13H2,1H3,(H,27,28,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Angiotensin II receptor antagonist activity was determined by 50% inhibition of specific binding of [3H]angiotensin II (2 nM) to rat adrenal cortical... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2T72KMB |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50281814
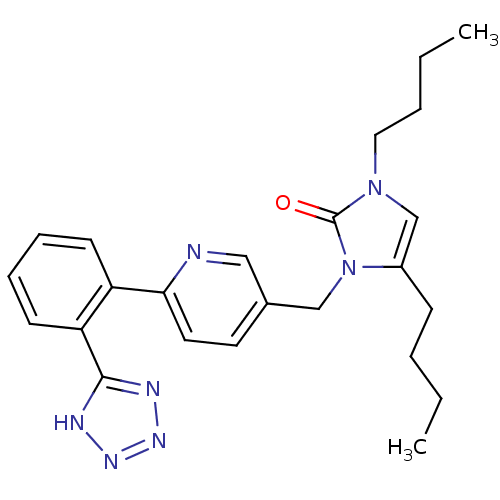
(1,4-Dibutyl-3-{6-[2-(1H-tetrazol-5-yl)-phenyl]-pyr...)Show SMILES CCCCc1cn(CCCC)c(=O)n1Cc1ccc(nc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H29N7O/c1-3-5-9-19-17-30(14-6-4-2)24(32)31(19)16-18-12-13-22(25-15-18)20-10-7-8-11-21(20)23-26-28-29-27-23/h7-8,10-13,15,17H,3-6,9,14,16H2,1-2H3,(H,26,27,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of [125I]-AII binding to Angiotensin II receptor of rat uterine membrane |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2JQ135P |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230796

(CHEMBL56690)Show SMILES CCCCc1ncc(\C=C(/Cc2cccs2)C(O)=O)n1Cc1ccc(cc1)-c1ccccc1C(O)=O Show InChI InChI=1S/C29H28N2O4S/c1-2-3-10-27-30-18-23(16-22(28(32)33)17-24-7-6-15-36-24)31(27)19-20-11-13-21(14-12-20)25-8-4-5-9-26(25)29(34)35/h4-9,11-16,18H,2-3,10,17,19H2,1H3,(H,32,33)(H,34,35)/b22-16+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50145967
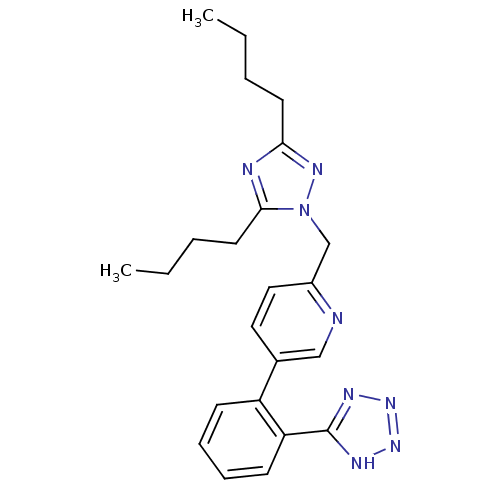
(2-(3,5-Dibutyl-[1,2,4]triazol-1-ylmethyl)-5-[2-(1H...)Show SMILES CCCCc1nc(CCCC)n(Cc2ccc(cn2)-c2ccccc2-c2nnn[nH]2)n1 Show InChI InChI=1S/C23H28N8/c1-3-5-11-21-25-22(12-6-4-2)31(28-21)16-18-14-13-17(15-24-18)19-9-7-8-10-20(19)23-26-29-30-27-23/h7-10,13-15H,3-6,11-12,16H2,1-2H3,(H,26,27,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Tested in vitro for inhibition of [125I]AII to rat uterine membranes |
Citation and Details
Article DOI: 10.1016/S0960-894X(01)81129-X
BindingDB Entry DOI: 10.7270/Q2PN98BK |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50049209
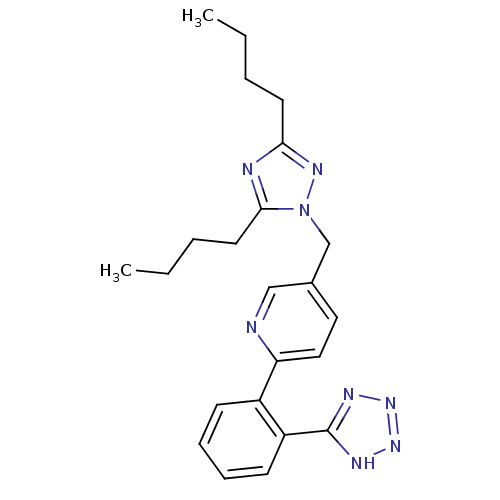
(5-(3,5-Dibutyl-[1,2,4]triazol-1-ylmethyl)-2-[2-(1H...)Show SMILES CCCCc1nc(CCCC)n(Cc2ccc(nc2)-c2ccccc2-c2nnn[nH]2)n1 Show InChI InChI=1S/C23H28N8/c1-3-5-11-21-25-22(12-6-4-2)31(28-21)16-17-13-14-20(24-15-17)18-9-7-8-10-19(18)23-26-29-30-27-23/h7-10,13-15H,3-6,11-12,16H2,1-2H3,(H,26,27,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of [125I]-AII binding to Angiotensin II receptor of rat uterine membrane |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2JQ135P |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50049209

(5-(3,5-Dibutyl-[1,2,4]triazol-1-ylmethyl)-2-[2-(1H...)Show SMILES CCCCc1nc(CCCC)n(Cc2ccc(nc2)-c2ccccc2-c2nnn[nH]2)n1 Show InChI InChI=1S/C23H28N8/c1-3-5-11-21-25-22(12-6-4-2)31(28-21)16-17-13-14-20(24-15-17)18-9-7-8-10-19(18)23-26-29-30-27-23/h7-10,13-15H,3-6,11-12,16H2,1-2H3,(H,26,27,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Tested in vitro for inhibition of [125I]AII to rat uterine membranes |
Citation and Details
Article DOI: 10.1016/S0960-894X(01)81129-X
BindingDB Entry DOI: 10.7270/Q2PN98BK |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
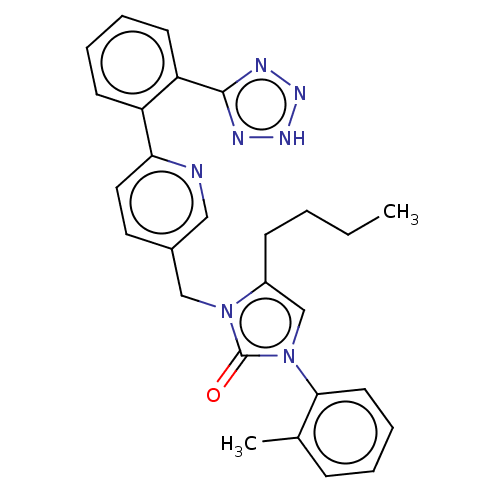
(RAT) | BDBM50212844
(CHEMBL276689)Show SMILES CCCCc1cn(-c2ccccc2C)c(=O)n1Cc1ccc(nc1)-c1ccccc1-c1nn[nH]n1 Show InChI InChI=1S/C27H27N7O/c1-3-4-10-21-18-34(25-13-8-5-9-19(25)2)27(35)33(21)17-20-14-15-24(28-16-20)22-11-6-7-12-23(22)26-29-31-32-30-26/h5-9,11-16,18H,3-4,10,17H2,1-2H3,(H,29,30,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of [125I]-AII binding to Angiotensin II receptor of rat uterine membrane |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2JQ135P |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50230772

(CHEMBL59043)Show SMILES CCCc1cccc(\C=C(/Cc2cccs2)C(O)=O)c1Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C25H24O4S/c1-2-5-18-6-3-7-20(15-21(25(28)29)16-22-8-4-13-30-22)23(18)14-17-9-11-19(12-10-17)24(26)27/h3-4,6-13,15H,2,5,14,16H2,1H3,(H,26,27)(H,28,29)/b21-15+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [125I]AII binding to Angiotensin II receptor, type 1 of rat mesenteric arteries |
J Med Chem 36: 1880-92 (1993)
BindingDB Entry DOI: 10.7270/Q2PK0JC7 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
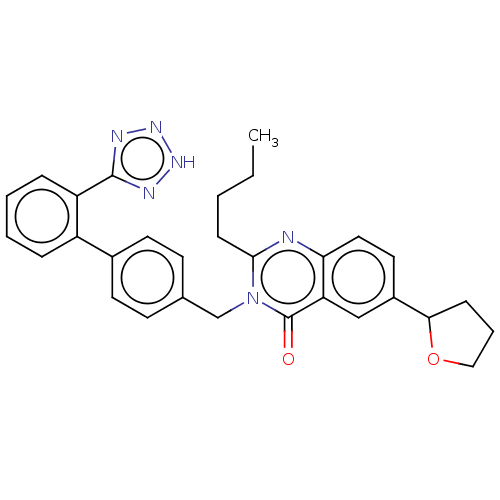
(RAT) | BDBM50212954
(CHEMBL295114)Show SMILES CCCCc1nc2ccc(cc2c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)C1CCCO1 Show InChI InChI=1S/C30H30N6O2/c1-2-3-10-28-31-26-16-15-22(27-9-6-17-38-27)18-25(26)30(37)36(28)19-20-11-13-21(14-12-20)23-7-4-5-8-24(23)29-32-34-35-33-29/h4-5,7-8,11-16,18,27H,2-3,6,9-10,17,19H2,1H3,(H,32,33,34,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro Angiotensin II receptor antagonist activity in rat adrenal cortex tissue. |
Bioorg Med Chem Lett 4: 1703-1708 (1994)
Article DOI: 10.1016/S0960-894X(00)80365-0
BindingDB Entry DOI: 10.7270/Q2JS9QB3 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50281822

(1,4-Dibutyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-yl...)Show SMILES CCCCc1cn(CCCC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C25H30N6O/c1-3-5-9-21-18-30(16-6-4-2)25(32)31(21)17-19-12-14-20(15-13-19)22-10-7-8-11-23(22)24-26-28-29-27-24/h7-8,10-15,18H,3-6,9,16-17H2,1-2H3,(H,26,27,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of [125I]-AII binding to Angiotensin II receptor of rat uterine membrane |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2JQ135P |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
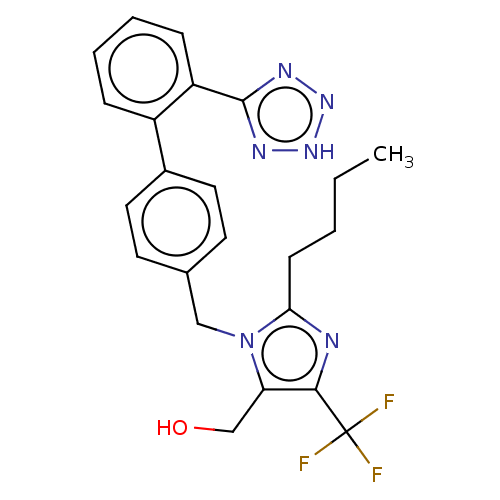
(RAT) | BDBM50229469
(CHEMBL315094)Show SMILES CCCCc1nc(c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)C(F)(F)F Show InChI InChI=1S/C23H23F3N6O/c1-2-3-8-20-27-21(23(24,25)26)19(14-33)32(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-28-30-31-29-22/h4-7,9-12,33H,2-3,8,13-14H2,1H3,(H,28,29,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of radioligand [3H]angiotensin II binding to angiotensin II receptor of rat adrenal cortical membrane |
J Med Chem 34: 2525-47 (1991)
BindingDB Entry DOI: 10.7270/Q2QN690P |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50226528
(CHEMBL284425)Show SMILES CCCCc1nc(Cl)c(COC)n1Cc1ccc(NC(=O)c2ccccc2S(O)(=O)=O)cc1 Show InChI InChI=1S/C23H26ClN3O5S/c1-3-4-9-21-26-22(24)19(15-32-2)27(21)14-16-10-12-17(13-11-16)25-23(28)18-7-5-6-8-20(18)33(29,30)31/h5-8,10-13H,3-4,9,14-15H2,1-2H3,(H,25,28)(H,29,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor |
J Med Chem 33: 1312-29 (1990)
BindingDB Entry DOI: 10.7270/Q2TM7DCT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM82258

(CAS_114798-26-4 | Losartan | NSC_3961)Show SMILES CCCCc1nc(Cl)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H23ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3,(H,25,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of Angiotensin II receptor binding of radiolabeled AII to rat adrenal glomerulosa tissue by 50% |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z03B9M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data