

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taste receptor type 1 member 1/3 (Homo sapiens (Human)) | BDBM50062279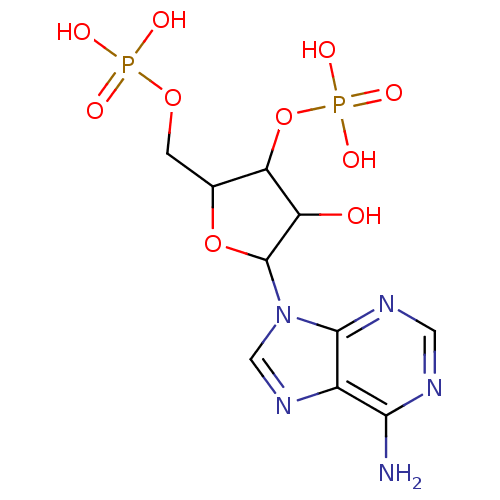 (CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...) | UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration of alanine is 20 mM. | Citation and Details BindingDB Entry DOI: 10.7270/Q2KP859X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Taste receptor type 1 member 1/3 (Homo sapiens (Human)) | BDBM50062279 (CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...) | UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration of alanine is 20 mM. | Citation and Details BindingDB Entry DOI: 10.7270/Q2KP859X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50062279 (CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Agonist activity at P2Y1 receptor measured as capacity to stimulate 50% phospholipase C in turkey erythrocyte membranes | J Med Chem 41: 183-90 (1998) Article DOI: 10.1021/jm970433l BindingDB Entry DOI: 10.7270/Q2SX6DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50062279 (CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.34E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Concentration at which 50% of the maximal effect (stimulation of PLC at P2Y1 receptor in the turkey erythrocyte membranes) is reached | J Med Chem 42: 1625-38 (1999) Article DOI: 10.1021/jm980657j BindingDB Entry DOI: 10.7270/Q28C9WZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Taste receptor type 1 member 1/3 (Homo sapiens (Human)) | BDBM50062279 (CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...) | UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration of alanine is 20 mM. | Citation and Details BindingDB Entry DOI: 10.7270/Q2KP859X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Taste receptor type 1 member 1/3 (Homo sapiens (Human)) | BDBM50062279 (CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...) | UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration of alanine is 20 mM. | Citation and Details BindingDB Entry DOI: 10.7270/Q2KP859X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Taste receptor type 1 member 1/3 (Homo sapiens (Human)) | BDBM50062279 (CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...) | UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration of alanine is 20 mM. | Citation and Details BindingDB Entry DOI: 10.7270/Q2KP859X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Taste receptor type 1 member 1/3 (Homo sapiens (Human)) | BDBM50062279 (CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...) | UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA | Assay Description HEK293 cells that stably express T1R3 and inducibly express T1R1 were exposed to nucleotide derivatives alone to activate the umami receptor. Activat... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KP859X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Taste receptor type 1 member 1/3 (Homo sapiens (Human)) | BDBM50062279 (CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...) | UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA | Assay Description The concentration of IMP is 0.2 mM. | Citation and Details BindingDB Entry DOI: 10.7270/Q2KP859X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Taste receptor type 1 member 1/3 (Homo sapiens (Human)) | BDBM50062279 (CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...) | UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
TBA | Assay Description HEK293 cells that stably express T1R3 and inducibly express T1R1 were exposed to nucleotide derivatives alone to activate the umami receptor. Activat... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KP859X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Taste receptor type 1 member 1/3 (Homo sapiens (Human)) | BDBM50062279 (CHEMBL416789 | Phosphoric acid mono-[5-(6-amino-pu...) | UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
TBA | Assay Description HEK293 cells that stably express T1R3 and inducibly express T1R1 were exposed to nucleotide derivatives alone to activate the umami receptor. Activat... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KP859X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||