

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aurora kinase A (Homo sapiens (Human)) | BDBM12984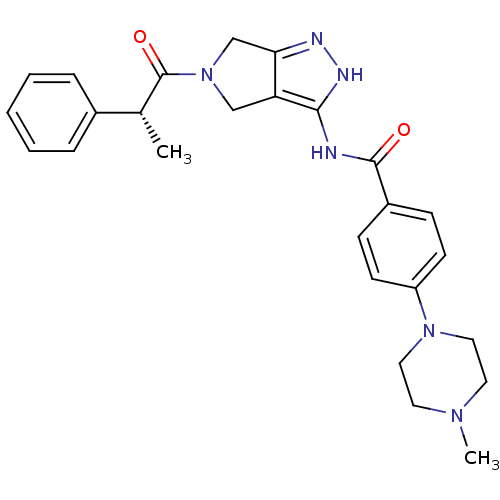 (4-(4-methylpiperazin-1-yl)-N-{5-[(2R)-2-phenylprop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | J Med Chem 49: 7247-51 (2006) Article DOI: 10.1021/jm060897w BindingDB Entry DOI: 10.7270/Q2NS0S4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM12984 (4-(4-methylpiperazin-1-yl)-N-{5-[(2R)-2-phenylprop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of aurora A kinase | J Med Chem 52: 2629-51 (2009) Checked by Author Article DOI: 10.1021/jm8012129 BindingDB Entry DOI: 10.7270/Q2B85920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM12984 (4-(4-methylpiperazin-1-yl)-N-{5-[(2R)-2-phenylprop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description Inhibition of aurora B kinase | J Med Chem 52: 2629-51 (2009) Checked by Author Article DOI: 10.1021/jm8012129 BindingDB Entry DOI: 10.7270/Q2B85920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM12984 (4-(4-methylpiperazin-1-yl)-N-{5-[(2R)-2-phenylprop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 99 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | J Med Chem 49: 7247-51 (2006) Article DOI: 10.1021/jm060897w BindingDB Entry DOI: 10.7270/Q2NS0S4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||