

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin K (Oryctolagus cuniculus (rabbit)) | BDBM19828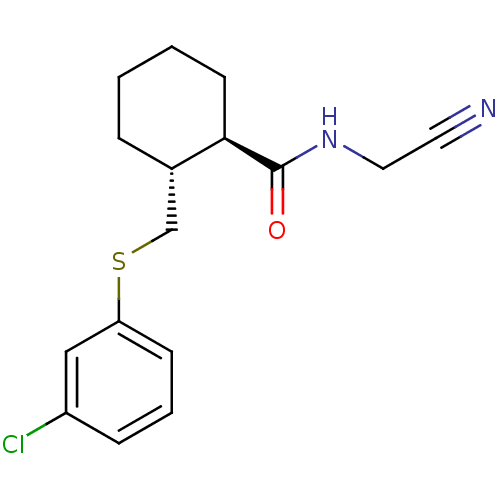 ((1R,2R)-2-{[(3-chlorophenyl)sulfanyl]methyl}-N-(cy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 49: 1066-79 (2006) Article DOI: 10.1021/jm051059p BindingDB Entry DOI: 10.7270/Q2DF6PH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19828 ((1R,2R)-2-{[(3-chlorophenyl)sulfanyl]methyl}-N-(cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universit£t Curated by ChEMBL | Assay Description Inhibition of cathepsin L | J Med Chem 55: 2932-42 (2012) Article DOI: 10.1021/jm201706b BindingDB Entry DOI: 10.7270/Q2RJ4KGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM19828 ((1R,2R)-2-{[(3-chlorophenyl)sulfanyl]methyl}-N-(cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universit£t Curated by ChEMBL | Assay Description Inhibition of cathepsin B | J Med Chem 55: 2932-42 (2012) Article DOI: 10.1021/jm201706b BindingDB Entry DOI: 10.7270/Q2RJ4KGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||