

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [600-1027,K702N]/[600-1155,K702N] (Human immunodeficiency virus type 1) | BDBM2104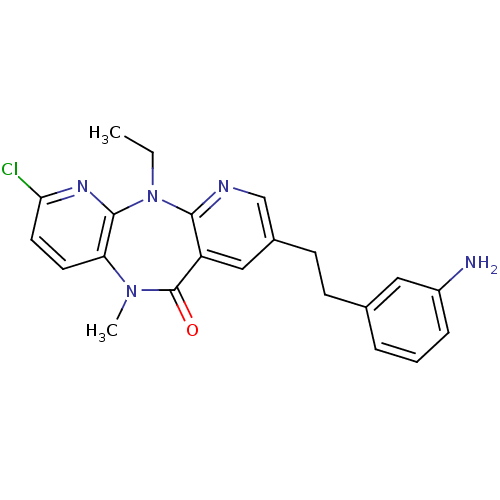 (13-[2-(3-aminophenyl)ethyl]-5-chloro-2-ethyl-9-met...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 41: 2960-71 (1998) Article DOI: 10.1021/jm9707028 BindingDB Entry DOI: 10.7270/Q28W3BH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,G789A]/[600-1155,G789A] (Human immunodeficiency virus type 1) | BDBM2104 (13-[2-(3-aminophenyl)ethyl]-5-chloro-2-ethyl-9-met...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 41: 2960-71 (1998) Article DOI: 10.1021/jm9707028 BindingDB Entry DOI: 10.7270/Q28W3BH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,P835L]/[600-1155,P835L] (Human immunodeficiency virus type 1) | BDBM2104 (13-[2-(3-aminophenyl)ethyl]-5-chloro-2-ethyl-9-met...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 41: 2960-71 (1998) Article DOI: 10.1021/jm9707028 BindingDB Entry DOI: 10.7270/Q28W3BH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,V705A]/[600-1155,V705A] (Human immunodeficiency virus type 1) | BDBM2104 (13-[2-(3-aminophenyl)ethyl]-5-chloro-2-ethyl-9-met...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 41: 2960-71 (1998) Article DOI: 10.1021/jm9707028 BindingDB Entry DOI: 10.7270/Q28W3BH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM2104 (13-[2-(3-aminophenyl)ethyl]-5-chloro-2-ethyl-9-met...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 41: 2960-71 (1998) Article DOI: 10.1021/jm9707028 BindingDB Entry DOI: 10.7270/Q28W3BH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,Y780C]/[600-1155,Y780C] (Human immunodeficiency virus type 1) | BDBM2104 (13-[2-(3-aminophenyl)ethyl]-5-chloro-2-ethyl-9-met...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 41: 2960-71 (1998) Article DOI: 10.1021/jm9707028 BindingDB Entry DOI: 10.7270/Q28W3BH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,Y787L]/[600-1155,Y787L] (Human immunodeficiency virus type 1) | BDBM2104 (13-[2-(3-aminophenyl)ethyl]-5-chloro-2-ethyl-9-met...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 41: 2960-71 (1998) Article DOI: 10.1021/jm9707028 BindingDB Entry DOI: 10.7270/Q28W3BH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||