

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2337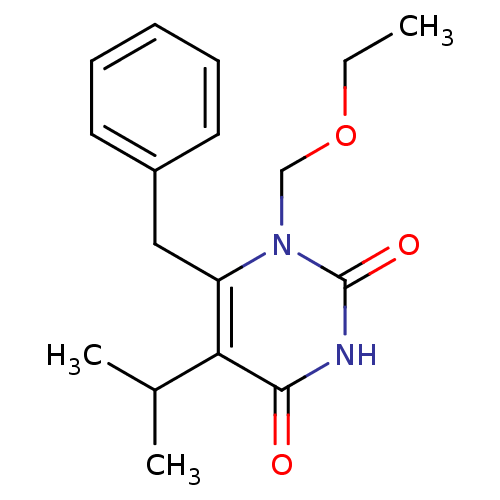 (6-benzyl-1-(ethoxymethyl)-5-(propan-2-yl)-1,2,3,4-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Oxford Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 reverse transcriptase | J Med Chem 39: 1589-600 (1996) Article DOI: 10.1021/jm960056x BindingDB Entry DOI: 10.7270/Q23X85QC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2337 (6-benzyl-1-(ethoxymethyl)-5-(propan-2-yl)-1,2,3,4-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Roma La Sapienza | Assay Description Inhibitory concentration required to inhibit the HIV-1 reverse transcriptase activity | J Med Chem 47: 928-34 (2004) Article DOI: 10.1021/jm0309856 BindingDB Entry DOI: 10.7270/Q26W988R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2337 (6-benzyl-1-(ethoxymethyl)-5-(propan-2-yl)-1,2,3,4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Universita degli Studi di Roma La Sapienza | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 47: 928-34 (2004) Article DOI: 10.1021/jm0309856 BindingDB Entry DOI: 10.7270/Q26W988R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2337 (6-benzyl-1-(ethoxymethyl)-5-(propan-2-yl)-1,2,3,4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Universita degli Studi di Roma La Sapienza | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 44: 2544-54 (2001) Article DOI: 10.1021/jm010853h BindingDB Entry DOI: 10.7270/Q2CN724V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2337 (6-benzyl-1-(ethoxymethyl)-5-(propan-2-yl)-1,2,3,4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Roma La Sapienza | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 42: 619-27 (1999) Article DOI: 10.1021/jm980260f BindingDB Entry DOI: 10.7270/Q2445JPQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2337 (6-benzyl-1-(ethoxymethyl)-5-(propan-2-yl)-1,2,3,4-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by ELISA | Bioorg Med Chem 18: 3231-7 (2010) Article DOI: 10.1016/j.bmc.2010.03.025 BindingDB Entry DOI: 10.7270/Q2057JQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2337 (6-benzyl-1-(ethoxymethyl)-5-(propan-2-yl)-1,2,3,4-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(ra)/oligo(dT)15 homopolymer template as substrate after 1 hr | J Med Chem 55: 2242-50 (2012) Article DOI: 10.1021/jm201506e BindingDB Entry DOI: 10.7270/Q22N554J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2337 (6-benzyl-1-(ethoxymethyl)-5-(propan-2-yl)-1,2,3,4-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of purified recombinant HIV-1 reverse transcriptase | Bioorg Med Chem Lett 9: 1593-8 (1999) BindingDB Entry DOI: 10.7270/Q27H1K3Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2337 (6-benzyl-1-(ethoxymethyl)-5-(propan-2-yl)-1,2,3,4-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibitory activity against recombinant HIV-1 reverse transcriptase (rRT) | Bioorg Med Chem Lett 9: 2721-6 (1999) BindingDB Entry DOI: 10.7270/Q2D50M49 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2337 (6-benzyl-1-(ethoxymethyl)-5-(propan-2-yl)-1,2,3,4-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibition of recombinant reverse transcriptase (RT) in cell-free Quan-T-RT assay system | Bioorg Med Chem Lett 9: 3411-6 (2000) BindingDB Entry DOI: 10.7270/Q2SX6CD8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||