

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3556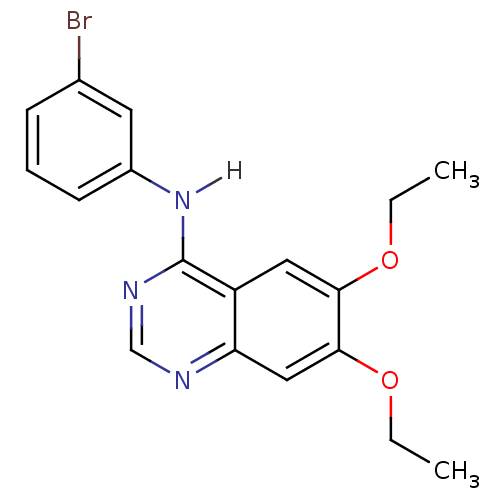 (4-[(3-Bromophenyl)amino]-6,7-diethoxyquinazoline |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 267-76 (1996) Article DOI: 10.1021/jm9503613 BindingDB Entry DOI: 10.7270/Q25T3HPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3556 (4-[(3-Bromophenyl)amino]-6,7-diethoxyquinazoline |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of wild type EGFR | Proc Natl Acad Sci USA 104: 20523-8 (2007) Checked by Author Article DOI: 10.1073/pnas.0708800104 BindingDB Entry DOI: 10.7270/Q2DB82RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3556 (4-[(3-Bromophenyl)amino]-6,7-diethoxyquinazoline |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.00603 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich Curated by ChEMBL | Assay Description Inhibition of EGFR | J Med Chem 51: 1179-88 (2008) Article DOI: 10.1021/jm070654j BindingDB Entry DOI: 10.7270/Q29Z95RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3556 (4-[(3-Bromophenyl)amino]-6,7-diethoxyquinazoline |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Displacement of [125I]4-(3-iodoanilino)-6,7-dimethoxyquinazoline from EGFR tyrosine kinase in human A431 cell membranes | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3556 (4-[(3-Bromophenyl)amino]-6,7-diethoxyquinazoline |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Inhibition of human EGFR tyrosine kinase phosphorylation expressed in mouse BaF3 cells | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-4 (Homo sapiens (Human)) | BDBM3556 (4-[(3-Bromophenyl)amino]-6,7-diethoxyquinazoline |...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Inhibition of human ErbB4 tyrosine kinase phosphorylation expressed in human CEM/4 cells | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM3556 (4-[(3-Bromophenyl)amino]-6,7-diethoxyquinazoline |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 215 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Inhibition of human ErbB2 tyrosine kinase phosphorylation expressed in mouse BaF3 cells | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-1,6-bisphosphatase 1 (Homo sapiens (Human)) | BDBM3556 (4-[(3-Bromophenyl)amino]-6,7-diethoxyquinazoline |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Concentration required to inhibit the human liver recombinant fructose-1,6-bisphosphatase. | Bioorg Med Chem Lett 11: 17-21 (2001) BindingDB Entry DOI: 10.7270/Q2HD7W52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-1,6-bisphosphatase 1 (Sus scrofa (Pig)) | BDBM3556 (4-[(3-Bromophenyl)amino]-6,7-diethoxyquinazoline |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Compound was evaluated for its concentration required to inhibit the porcine kidney F16BPase | Bioorg Med Chem Lett 11: 17-21 (2001) BindingDB Entry DOI: 10.7270/Q2HD7W52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-1,6-bisphosphatase 1 (Rattus norvegicus) | BDBM3556 (4-[(3-Bromophenyl)amino]-6,7-diethoxyquinazoline |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Compound was evaluated for its concentration required to inhibit the rat liver F16BPase | Bioorg Med Chem Lett 11: 17-21 (2001) BindingDB Entry DOI: 10.7270/Q2HD7W52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||