 Found 12 hits of ic50 for monomerid = 50026752
Found 12 hits of ic50 for monomerid = 50026752 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50026752
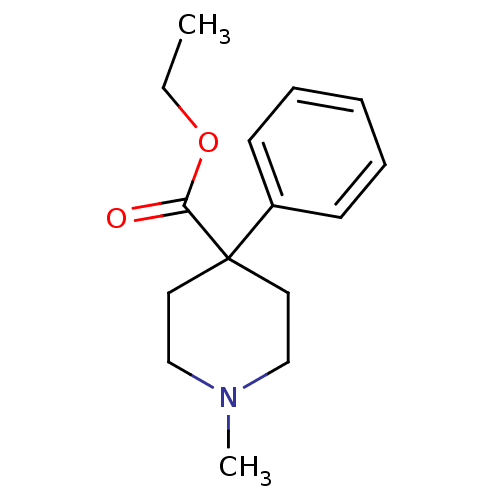
(1-Methyl-4-phenyl-piperidine-4-carboxylic acid eth...)Show InChI InChI=1S/C15H21NO2/c1-3-18-14(17)15(9-11-16(2)12-10-15)13-7-5-4-6-8-13/h4-8H,3,9-12H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 315 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu-opiate receptor (human) using [3H]DAMGO radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50026752

(1-Methyl-4-phenyl-piperidine-4-carboxylic acid eth...)Show InChI InChI=1S/C15H21NO2/c1-3-18-14(17)15(9-11-16(2)12-10-15)13-7-5-4-6-8-13/h4-8H,3,9-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 324 | n/a | n/a | n/a | n/a | n/a | n/a |
Reverse proteomics research institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against potassium channel HERG |
Bioorg Med Chem Lett 15: 2886-90 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.080
BindingDB Entry DOI: 10.7270/Q29S1S7C |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1
(Mus musculus (Mouse)-MOUSE) | BDBM50026752

(1-Methyl-4-phenyl-piperidine-4-carboxylic acid eth...)Show InChI InChI=1S/C15H21NO2/c1-3-18-14(17)15(9-11-16(2)12-10-15)13-7-5-4-6-8-13/h4-8H,3,9-12H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Duphar Research Laboratories
Curated by ChEMBL
| Assay Description
Tested for displacement of radioligand [3H]-Naloxone from opiate receptor |
J Med Chem 36: 2751-60 (1993)
BindingDB Entry DOI: 10.7270/Q2CZ367F |
More data for this
Ligand-Target Pair | |
Delta-type/Kappa-type/Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50026752

(1-Methyl-4-phenyl-piperidine-4-carboxylic acid eth...)Show InChI InChI=1S/C15H21NO2/c1-3-18-14(17)15(9-11-16(2)12-10-15)13-7-5-4-6-8-13/h4-8H,3,9-12H2,1-2H3 | PDB
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Opioid receptors with [3H]naloxone binding in the absence of NaCl |
J Med Chem 23: 985-90 (1980)
BindingDB Entry DOI: 10.7270/Q2HD7XT0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50026752

(1-Methyl-4-phenyl-piperidine-4-carboxylic acid eth...)Show InChI InChI=1S/C15H21NO2/c1-3-18-14(17)15(9-11-16(2)12-10-15)13-7-5-4-6-8-13/h4-8H,3,9-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 2.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 using [3H]- U-69,593 radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Delta-type/Kappa-type/Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50026752

(1-Methyl-4-phenyl-piperidine-4-carboxylic acid eth...)Show InChI InChI=1S/C15H21NO2/c1-3-18-14(17)15(9-11-16(2)12-10-15)13-7-5-4-6-8-13/h4-8H,3,9-12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]naloxone from Sprague-Dawley rat cerebellum opioid receptor assessed as relative receptor affinity by scintillation counting |
J Med Chem 21: 600-6 (1978)
Article DOI: 10.1021/jm00205a003
BindingDB Entry DOI: 10.7270/Q2WM1HDV |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50026752

(1-Methyl-4-phenyl-piperidine-4-carboxylic acid eth...)Show InChI InChI=1S/C15H21NO2/c1-3-18-14(17)15(9-11-16(2)12-10-15)13-7-5-4-6-8-13/h4-8H,3,9-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against delta-opiate receptor (human) using [3H]-DPDPE radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Delta-type/Kappa-type/Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50026752

(1-Methyl-4-phenyl-piperidine-4-carboxylic acid eth...)Show InChI InChI=1S/C15H21NO2/c1-3-18-14(17)15(9-11-16(2)12-10-15)13-7-5-4-6-8-13/h4-8H,3,9-12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]naloxone from Sprague-Dawley rat cerebellum opioid receptor assessed as relative receptor affinity by scintillation counting |
J Med Chem 21: 600-6 (1978)
Article DOI: 10.1021/jm00205a003
BindingDB Entry DOI: 10.7270/Q2WM1HDV |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 1
(Homo sapiens (Human)) | BDBM50026752

(1-Methyl-4-phenyl-piperidine-4-carboxylic acid eth...)Show InChI InChI=1S/C15H21NO2/c1-3-18-14(17)15(9-11-16(2)12-10-15)13-7-5-4-6-8-13/h4-8H,3,9-12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University Medicine Greifswald
Curated by ChEMBL
| Assay Description
Inhibition of human OCT1 expressed in HEK293 cells assessed as reduction in ASP+ substrate uptake by microplate reader based analysis |
J Med Chem 62: 9890-9905 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01301
BindingDB Entry DOI: 10.7270/Q2QV3QSW |
More data for this
Ligand-Target Pair | |
Delta-type/Kappa-type/Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50026752

(1-Methyl-4-phenyl-piperidine-4-carboxylic acid eth...)Show InChI InChI=1S/C15H21NO2/c1-3-18-14(17)15(9-11-16(2)12-10-15)13-7-5-4-6-8-13/h4-8H,3,9-12H2,1-2H3 | PDB
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Opioid receptors binding by inhibiting specific [3H]naloxone binding by 50% in the presence of 100 mM NaCl |
J Med Chem 23: 985-90 (1980)
BindingDB Entry DOI: 10.7270/Q2HD7XT0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50026752

(1-Methyl-4-phenyl-piperidine-4-carboxylic acid eth...)Show InChI InChI=1S/C15H21NO2/c1-3-18-14(17)15(9-11-16(2)12-10-15)13-7-5-4-6-8-13/h4-8H,3,9-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TCG Lifesciences Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Eur J Med Chem 46: 618-30 (2011)
Article DOI: 10.1016/j.ejmech.2010.11.042
BindingDB Entry DOI: 10.7270/Q2WQ052W |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50026752

(1-Methyl-4-phenyl-piperidine-4-carboxylic acid eth...)Show InChI InChI=1S/C15H21NO2/c1-3-18-14(17)15(9-11-16(2)12-10-15)13-7-5-4-6-8-13/h4-8H,3,9-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel in HEK293 cells by voltage-clamp method |
Eur J Med Chem 43: 2479-88 (2008)
Article DOI: 10.1016/j.ejmech.2007.12.025
BindingDB Entry DOI: 10.7270/Q2542PTB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data